2017 Volume 93 Issue 4 Pages 183-195
2017 Volume 93 Issue 4 Pages 183-195
Lymphocyte recirculation between the blood and the lymphoid/non-lymphoid tissues is an essential homeostatic mechanism that regulates humoral and cellular immune responses in vivo. This system promotes the encounter of naïve T and B cells with their specific cognate antigen presented by dendritic cells, and with the regulatory cells with which they need to interact to initiate, maintain, and terminate immune responses. The constitutive lymphocyte trafficking is mediated by particular types of blood vessels, including the high endothelial venules (HEVs) in lymph nodes and Peyer’s patches, and the flat-walled venules in non-lymphoid tissues including the skin. The lymphocyte migration across HEVs involves tethering/rolling, arrest/firm adhesion/intraluminal crawling, and transendothelial migration. On the other hand, relatively little is known about how lymphocytes and other types of cells migrate across the venules of non-lymphoid tissues. Here we summarize recent findings about the molecular mechanisms that govern immune cell trafficking, including the roles of chemokines and lysophospholipids in regulating immune cell motility and endothelial permeability.
The lymphoid system is composed of many different cell types distributed in various tissues, but it functions as a single entity. This cohesion is possible primarily because immune cells are mobile; among them, large numbers of lymphocytes traffic continually in a highly regulated manner between secondary lymphoid tissues, where antigens, antigen-presenting cells, immunocompetent lymphocytes, and regulatory cells are located.1)–3)
Under physiological conditions, naïve (i.e., mature, non-activated) lymphocytes migrate from the peripheral blood into lymph nodes and Peyer’s patches by selectively interacting with the specialized endothelium of postcapillary venules called high endothelial venules (HEVs). HEVs are distinctive microvascular segments located mainly in the interfollicular area of these tissues and consist of tall and cuboidal endothelial cells (ECs) surrounded by a thick basal lamina and a prominent perivascular sheath. A remarkable feature of HEVs is that their ECs allow numerous lymphocytes to adhere to and migrate between EC junctions, during which both cell types display markedly motile changes.4)
Flat-walled venules in non-lymphoid tissues including the skin also support leukocyte extravasation under physiological conditions, but unlike HEVs, they allow mainly memory-type lymphocytes and dendritic cells (DCs) to extravasate,1)–3) and the extent of extravasation is much smaller than that observed with HEVs. Relatively little is known about the molecular mechanisms underlying leukocyte extravasation through the flat-walled venules, although both adhesion molecules and chemokines appear to play important roles, as is the case with HEVs.
In the early 1980s, Yamaguchi and Schoefl documented that circulating lymphocytes are able to selectively recognize and adhere to the lumen of HEVs and that this mechanism is influenced by the circulating lymphocyte level.5) They found through meticulous electron microscopic analyses that, whereas about 40% of the lymphocytes interacting with the HEV ECs are in the process of transmigrating in normal mice, about 90% of the interacting cells are in the process of transmigrating in lymphopenic mice in which circulating lymphocytes were depleted by thoracic duct cannulation,5) indicating that lymphocyte transmigration is upregulated when the circulating lymphocyte level is low. They also found in the lymphocyte-depleted mice that intravenously injected lymphocytes swiftly adhere to HEVs and penetrate the HEV wall (Fig. 1) and that the speed and intensity of lymphocyte binding/transmigration are both greatly enhanced compared with normal mice.5) These observations indicated that lymphocyte trafficking across HEVs is homeostatically regulated by the number of lymphocytes in the blood. It could be that a humoral factor(s) produced under lymphopenic conditions acts on lymphocytes and/or HEV ECs to enhance lymphocyte transmigration.

Lymphocyte transmigration across HEVs is regulated homeostatically. Left; Scanning electron micrograph (SEM) of a sectioned HEV of a normal mouse, showing numerous lymphocytes adhering to endothelial cells before migrating between them (Courtesy of Dr. K. Yamaguchi, Yamaguchi University School of Medicine). Three of them (indicated by asterisk) are in the process of transmigrating, as judged by their reduced cell size and microvilli. Right; SEM of a sectioned HEV of a mouse that was subjected to lymphocyte depletion by chronic thoracic duct cannulation and then injected with lymphocytes intravenously. Scale bar: 10 µm. The injected lymphocytes quickly adhered to the endothelial surface and started penetrating the endothelial wall; all of the cells in the field are in the process of transmigrating (a transmigrating lymphocyte at the top left is magnified in the inset at the bottom right). These observations show that lymphocyte transmigration is markedly upregulated when the circulating lymphocyte level is low.
It was subsequently found that the lymphocytes’ interaction with HEV ECs is directed by a site-specific adhesion cascade involving several specific molecules and chemokines that act in sequence (Fig. 2).1),2),6) This adhesion cascade is initiated by leukocyte tethering/rolling, followed by the firm arrest of rolling lymphocytes, and finally by the transvenular migration (extravasation) of lymphocytes.2),3),7) This entire process is very similar to that observed in the lumen of inflamed blood vessels,8) although some distinct and specific molecules are used in HEVs, as detailed below.
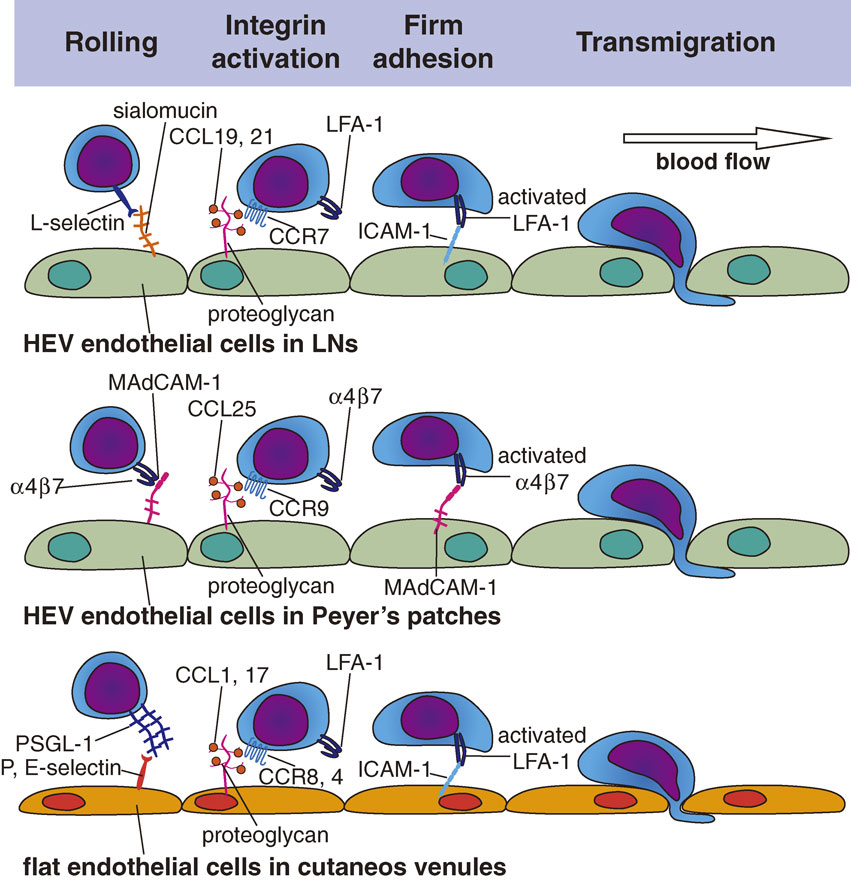
The lymphocyte adhesion cascade in the lumen of HEVs and cutaneous venules. In peripheral lymph node HEVs, lymphocytes initiate rolling through L-selectin–sialomucin interactions. Subsequently, the LFA-1 integrin on T cells is activated mainly by chemokines (CCL21 and CCL19). The T cells then bind to EC molecules such as ICAM-1 through the activated LFA-1, and proceed to migrate across HEVs. In Peyer’s patch HEVs, lymphocyte rolling is mediated by L-selectin–sialomucin interactions and α4β7 integrin–MAdCAM-1 interactions. The chemokines that trigger integrin activation in T and B cells appear to be the same as in lymph nodes. In cutaneous post-capillary venules, T cell rolling, integrin activation and cell adhesion are regulated by PSGL-1-selectins interactions, chemokines including CCL1 and CCL17, and LFA1-ICAM-1 interactions, respectively.
As naïve lymphocytes flow into HEVs, they decelerate rapidly and exhibit weak, transient, on-and-off adhesive interactions (tethering) with the HEV ECs and roll along the inner surface of the HEV wall (rolling). This process is mediated by CD62L (L-selectin) on lymphocytes and by sialomucins expressed on HEV ECs (Fig. 2). L-selectin is a lectin-type cell adhesion molecule that recognizes sugars and is expressed on all leukocytes. It binds to specific O-glycans expressed on HEV sialomucins, a group of heavily glycosylated proteins (mucins) whose carbohydrate moieties contain sialic acid. The critical recognition determinant on the O-glycans is 6-sulfo sialyl Lewis X (sLex), which serves as a capping structure on core-2 and extended core-1 branches, and is specifically recognized by the MECA-79 monoclonal antibody. HEVs in the peripheral lymph nodes express at least five different sialomucins, including GlyCAM-1,9) CD34,10) podocalyxin,11) endomucin,12) and nepmucin/CD300g.13) To synthesize the L-selectin-binding MEC-79-reactive sLex structures, HEV ECs express a set of glycosyltransferases, including α1,3-fucosyltransferases IV and VII, Core1-β3GlcNAcT (also known as β3GlcNAcT-3 or Core1-GlcNAcT), Core2-β1,6-GlcNAcT (Core2-GlcNAcT), GlcNAc6ST-1, and GlcNAc6ST-2.14)
The HEV sialomucins are also called peripheral node addressins (PNAds), because they function as “address code” molecules for peripheral node HEVs. These sialomucins share a common sugar epitope, i.e., the 6-sulfo sLex structure mentioned above. Binding of the MECA-79 antibody to this epitope abrogates L-selectin-PNAd interactions. In mesenteric lymph node HEVs, MAdCAM-1, a sialomucin bearing two immunoglobulin-like domains,15) serves as a vascular addressin, and α4β7 integrin serves as the cognate lymphocyte receptor that mediates rolling and adhesion,16) as described below. Within the HEV lumen, all L-selectin-expressing leukocytes undergo rolling/tethering, but as described below, only lymphocytes show firm adhesion to HEV ECs2) (Fig. 2).
ii) Firm arrest/adhesion.After tethering/rolling, lymphocytes further decelerate and undergo a shear-resistant firm arrest/adhesion to the HEV wall, which is primarily mediated by an interaction between the β2 integrin LFA-1 (CD11a/CD18) on lymphocytes and ICAM-1/ICAM-2 on HEV ECs (Fig. 2). While L-selectin is constitutively active, integrins generally need to be activated to mediate adhesion. This integrin activation is induced by G-protein coupled receptor (GPCR) signaling in the lymphocytes at HEVs, which is initiated by the lymphocytes’ interaction with chemokines displayed on ECs via specific receptors. Chemokines are generally positively charged, and hence bind to negatively charged molecules such as certain glycosaminoglycan chains that are abundantly expressed on HEV ECs; this binding prevents the chemokines from getting washed off by the blood flow.2),17) In the peripheral lymph nodes, LFA-1 on T cells is activated when HEV-associated chemokines including CCL21/CCL19 and CXCL12 interact with their specific receptors on T cells, CCR7 and CXCR4, respectively, whereas LFA-1 on B cells is activated by interactions with CXCL13 via CXCR5. Because these chemokine receptors are expressed preferentially on lymphocytes, only lymphocytes exhibit firm adhesion via activated LFA-1, which then binds to the immunoglobulin-like-domain-containing adhesion molecules, ICAM-1 and ICAM-2 on the surface of HEV ECs.
iii) Intraluminal crawling and transmigration.Upon undergoing firm adhesion, naïve lymphocytes start to crawl along the luminal surface of HEVs (intraluminal crawling) and slowly migrate to distant emigration sites, where they then transmigrate at certain hot spots (“exit ramps”)4) along the HEV wall (Fig. 2). The HEV basal lamina has numerous pores, through which lymphocytes pass to reach the abluminal side of HEVs, without disrupting the basal lamina. The basal lamina consists of type IV collagen, fibronectin, and laminin, which allow chemokine immobilization mainly via electrostatic interactions. Hence, the HEV basal lamina binds locally produced lymphoid chemokines, including CCL21, CCL19, CXCL12, and CXCL13, creating a chemokine-rich environment. The HEV basal lamina also functions as a guidance structure for the directional trafficking of lymphocytes from HEVs into the lymphoid tissue parenchyma.2)
Although lymphocytes were reported to cross the endothelial barrier using both paracellular (between adjacent ECs) and transcellular (through the cytoplasm of ECs) migration routes, Schoefl clearly showed, using electron microscopic and mathematical analyses, that lymphocytes predominantly use the paracellular route to exit HEVs.18)
In addition to the mechanisms described above, a variety of other adhesion molecules expressed on HEV ECs have been implicated in lymphocyte transmigration, although their modes of action remain ill defined. These molecules include CD31, VCAM-1, JAM-A, JAM-B, JAM-C, ESAM, VE-cadherin, and nepmucin (CD300g).19)
A specific lysophospholipid, lysophosphatidic acid (LPA) plays a particularly important role in the lymphocyte transmigration across HEVs. LPA is a naturally occurring bioactive lysophospholipid, consisting of a phosphate, a glycerol, and a fatty acid (Fig. 3a). LPA is mainly derived from its precursor, lysophosphatidylcholine, which is abundant in plasma, by the enzymatic action of the lysophospholipase D, autotaxin (ATX). When LPA binds to one of its specific receptors, LPA1–LPA6, which are all GPCRs, multiple signaling pathways are activated with various downstream physiological and pathological effects. Approximately ten years ago, we20) and Steve Rosen’s group21) found that the primary LPA-producing enzyme ATX is transcribed abundantly in HEV ECs. Subsequent analyses showed that the ATX expressed in HEVs regulates lymphocyte trafficking by locally generating LPA. Local inhibition of the ATX/LPA axis substantially blocks the lymphocyte transmigration across HEVs, while a local administration of LPA abrogates this effect.22) At the HEV lumen, LPA acts directly on HEV ECs via its receptors LPA4 and LPA6, although its actions through these receptors are different, given that LPA4 deficiency causes delayed lymphocyte transmigration across the HEV wall, whereas LPA6 deficiency also compromises lymphocyte transmigration but to a much lesser extent.23) In an in vitro transmigration assay, ATX inhibition impairs the release of lymphocytes that migrate underneath HEV ECs, and this defect is abrogated by adding LPA; LPA appears to contribute to lymphocyte de-adhesion (or release) from ECs by regulating the myosin II activity in HEV ECs (Fig. 3b).22)

A specific lysophospholipid, LPA, promotes lymphocyte extravasation from HEVs and lymphocyte motility in the lymph node parenchyma. (a) Structure of LPA. LPA has a phosphate group, a glycerol backbone, and a single fatty acid chain. (b) In the vicinity of HEVs, ATX is expressed at high levels and generates LPA locally. LPA in turn acts on HEV ECs via LPA4 and LPA6 to increase their motility, promoting dynamic lymphocyte-HEV interactions and subsequent lymphocyte de-adhesion from HEV ECs at the steady state. (c) In the lymph node cortex, ATX is expressed by fibroblastic reticular cells and generates LPA locally. LPA acts on lymphocytes via LPA2 to optimize T-cell movement, allowing the cells to navigate the highly confined environment in the lymph node parenchyma.
Under physiological conditions, not only naïve lymphocytes but also DC precursors, plasmacytoid DCs (pDCs), and central memory T cells extravasate from HEVs. While the DC precursor migration into lymph nodes is thought to follow basically the same steps as that of naïve lymphocytes,24) its precise mechanism remains to be fully explored. The pDCs show robust transmigration underneath HEV ECs but not non-HEV ECs, using adhesion molecules very similar to those used by naïve lymphocytes.25) The pDCs also require CCR7 to enter the lymph nodes via HEVs.26),27) This is also the case for central memory T cells, which readily proliferate and differentiate into effector cells in response to their antigenic stimulation in lymph nodes. These cells characteristically express high levels of L-selectin and CCR7, which they use to interact with HEV ECs, just like naïve T cells do. However, to what extent these cells require the HEV-associated lysophospholipid LPA for their transmigration remains to be explored.
When sterile inflammation occurs locally, blood-borne neutrophils rapidly and abundantly migrate into the draining lymph nodes via HEVs. Under these conditions, IL-17-producing lymphocytes first migrate into the draining lymph nodes, where they produce IL-17, which induces the production of CXCL2, a chemokine ligand for CXCR2, in HEVs, leading to the HEV-mediated migration of CXCR2-expressing neutrophils from the blood into the draining lymph nodes. The effect of IL-17 on CXCL2 depends on IL-1β, which is also enhanced by IL-17.28) Thus, although neutrophils are prevented from entering lymph nodes via HEVs under physiological conditions, they can migrate into lymph nodes abundantly when HEVs undergo an inflammation-induced molecular switch, which initiates the neutrophils’ CXCR2 engagement by CXCL2 displayed on ECs.
B) HEVs in intestinal lymphoid tissues.The lymphocyte trafficking to the small intestine is governed by two types of adhesion pathways. One is mediated by the interaction between lymphocyte L-selectin and HEV-expressed sialomucins/PNAds, which is mainly used by naïve lymphocytes, and the other is mediated by the interaction between lymphocyte integrin α4β7 and the vascular cell adhesion molecule MAdCAM-1, which is mainly used by lymphocytes that have been exposed to antigen-experienced DCs in the small intestine. When naïve lymphocytes that have migrated into the small intestine encounter DCs, they are exposed to high concentrations of DC-derived retinoic acid and start to upregulate their expressions of the integrin α4β7 and the chemokine receptor CCR9.29) The α4β7 specifically binds MAdCAM-1, and CCR9 is the receptor for the chemokine CCL25 secreted by small intestinal venules. Thus, these lymphocytes use α4β7 and CCR9 to recognize tissue-specific cues expressed on small intestinal ECs, i.e., MAdCAM-1 and CCL25. Recently, the orphan chemokine receptor GPR15 has been shown to control the localization of T effector cells in the colon.30)
C) Flat ECs in peripheral tissues.Flat ECs found in non-specialized regular postcapillary venules in the skin also mediate constitutive immune cell trafficking under steady state conditions, albeit to a much lesser extent than do HEV ECs. These ECs support leukocyte rolling under non-inflamed conditions,31) due to a constitutive, low-level expression of E- and P-selectins. The rolling frequency is largely determined by P-selectin, with E-selectin playing a smaller role, and L-selectin is not involved.32) Most skin T cells express an E- and P-selectin–binding molecule, the cutaneous lymphocyte antigen (CLA), which is derived from the glycosylation of a lymphocyte sialomucin PSGL-1, and a chemokine receptor CCR8, whose expression is induced by keratinocyte-derived factors.33) Upon binding to EC-displayed selectins, the CLA-expressing T cells extravasate from dermal venules. The engagement of lymphocyte CCR8 with its ligand CCL1 constitutively expressed in the dermal venules is thought to promote this extravasation by activating lymphocyte integrins. Skin T cells also express another chemokine receptor CCR4, whose engagement with a dermal venule-expressed chemokine, CCL17, promotes T cell migration into the skin. In inflamed skin, activated T cells express high levels of CCR10, whose engagement with CCL27, which is produced by keratinocytes and is highly displayed on inflamed venules in the skin, is critical for T cell recruitment to the skin.34) Interestingly, skin DCs are able to produce the active vitamin D3 metabolite 1,25(OH)2D3 from sunlight-induced vitamin D3, and 1,25(OH)2D3 upregulates the CCR10 expression in T cells.35) However, CCR10 is largely absent from the T cells in uninflamed skin;33),36) hence, the CCL17/CCR10 axis appears to be important for T cell migration into inflamed skin but not for the constitutive T cell migration into normal skin.
Recent studies indicate that T cells are abundant in the skin and have unique immunological abilities.37) A careful study in humans indicated that 2 × 1010 T cells exist in the skin, which is almost twice the number of T cells in the entire circulation.38) Most of the skin T cells have the phenotype of effector memory T cells, and some are central memory T cells. The prevailing hypothesis is that effector memory T cells that arise upon the antigenic stimulation of naïve as well as central memory T cells in lymph nodes migrate into peripheral tissues via venules bearing flat ECs and return to the lymph nodes via lymphatics, whereas the central memory T cells that also arise in lymph nodes recirculate between the blood vascular and lymphatic vascular systems using HEVs, just like naïve T cells.39),40) However, the presence of CLA-expressing central memory (L-selectin+CCR7+) T cells in the skin (∼20% of normal skin T cells) in humans41) indicates that not all of the central memory T cells recirculate via the conventional route; some of them migrate into the periphery and then move to the lymph nodes via lymphatics. On the other hand, a substantial proportion of memory T cells in the skin appear to be sessile and do not leave the tissue; hence, they are now called resident memory T cells (TRMs).42) These cells provide effective protection against local antigen re-challenge. The failure of these cells to exit the tissue is currently thought to be due to their low expression of the transcription factor KLF2 and of S1PR1 and to their high expression of the C-type lectin CD69; as a result of these conditions, the cells fail to respond to an “exit cue” provided by S1P (sphingosine-1-phosphate), which is released mainly from lymphatic ECs. S1P’s involvement in lymphocyte egress will be discussed below.
Regulatory T cells (Tregs) are also found in the skin, where they comprise 10∼20% of the normal skin T cells in man38) and mouse.43) They constitutively migrate to the draining lymph nodes via lymphatics in the steady state.44) These cells show increased migration during cutaneous immune responses and return to the skin upon re-exposure to antigen. The migrating Tregs have a stronger immunosuppressive effect than lymph node-residing Tregs and appear to contribute to the downregulation of cutaneous immune responses.44) These Tregs express CD103, CCR4, and CCR5, but the molecular mechanism underlying their migration from the skin to lymph nodes remains unclear.
A recent study using transgenic mice expressing a photoconvertible fluorescent protein, Kaede, confirmed that DCs also continuously migrate from the skin to draining lymph nodes under steady-state conditions.44) DCs do not apparently require β2 integrins for their migration, since CD18−/− mice deficient in the β2 integrin subunit show uncompromised DC migration from the blood to normal or inflamed skin, or from the skin to draining lymph nodes.45) This finding was later verified by multiphoton microscopy, which showed that DCs crawl into lymphatics independent of integrins46) and that they are guided into lymphatics by a tissue-immobilized gradient of CCL21 in an integrin-independent but CCR7-dependent manner.47)
Innate lymphoid cells (ILCs) are a group of non-T, non-B lymphocytes that are important in innate immune responses and in the regulation of inflammation.48) In the skin, group 2 ILCs are relatively abundant and continuously patrol the tissue at speeds comparable to those described for dermal DCs, frequently interacting with perivascular mast cells.49) However, how these cells are recruited to the skin and whether they migrate from the skin into the draining lymph nodes remain unknown.
The trafficking of lymphocytes and DCs into the lymphatic vessels is an active process.50) Chemokines presented on lymphatic ECs are important for attracting and guiding DCs into lymphatics, and DCs interacting with the lymphatic ECs respond to these chemokines. For instance, a CCR7-ligand chemokine, CCL21, is abundantly expressed on lymphatic capillaries and induces the chemotaxis of CCR7-expressing DCs, allowing them to migrate toward and to enter into lymphatics. CCL21 binds a lymphatic EC marker podoplanin with high affinity, is expressed on the basal lamina of lymphatics, and is shed into the perivascular stroma,51) which may contribute to the formation of a peri-lymphatic CCL21 concentration gradient. In addition, a chemokine-scavenging molecule, CCRL1, is expressed by lymphatic ECs that line the ceiling but not the floor of the subcapsular sinus.52) CCRL1 sequesters and induces CCL21 degradation, which is thought to contribute to the formation of a CCL21 concentration gradient from the sinus toward the lymph node parenchyma, which helps direct DCs to migrate toward the inner areas of lymph nodes. Indeed, DCs have been shown to require the lymphatic EC-displayed CCL21 to enter the lumen of lymphatics, in a CCR7-dependent manner.53),54)
In addition, CCR7’s function can be modulated locally; CCR7 is upregulated by locally generated molecules including prostaglandin E255) and extracellular NAD+,56) both of which are released from damaged or inflamed cells. Notably, however, while DC migration across the subcapsular sinus lymphatic EC layer is CCR7-dependent, T cell migration across the sinus lymphatic ECs is completely independent of CCR7 signaling.57) Thus, the CCL21-CCR7 axis does not appear to be the only regulator of immune cell trafficking across the lymph node subcapsular sinus.
Under inflamed conditions, the chemokine CX3CL1 (fractalkine) appears on lymphatic ECs and is actively secreted, and the soluble rather than membrane-anchored chemokine promotes DC migration toward lymphatics and EC transmigration.58) CXCL12 is also induced on the surface of lymphatic ECs upon an inflammatory stimulus, and DCs migrate across these cells in a CXCL12/CXCR4-dependent manner.59) In the case of lymphocytes, CXCL12 acts in synergy with CCR7 ligands to promote cell migration by sensitizing the cells through CXCR4, thus enabling them to respond to lower concentrations of CCR7 ligands.60) Given that mature DCs also express both CCR7 and CXCR4 at levels comparable to those on lymphocytes, chemokine-induced synergy may also enhance DC recruitment into lymphatics under certain conditions.
Interestingly, CCL1 is expressed by the subcapsular sinus lymphatic ECs of skin-draining lymph nodes but not by capillary lymphatic vessels in the skin, and inhibiting the CCL1/CCR8 interaction leads to impaired DC migration into the lymph node parenchyma, indicating that the CCL1/CCR8 axis functions downstream of the DC entry into lymphatics by regulating entry into the subcapsular sinus of the lymph nodes.61) Notably, malignant melanoma cells often express high levels of CCR8 and use CCL1-CCR8 to enter into the lymph node parenchyma, forming lymph node metastases.62) Thus, certain types of tumor cells can coopt the normal mechanism of CCL1-CCR8-dependent immune cell trafficking to metastasize to the lymph node.
b) Adhesion molecules and their receptors.As mentioned above, integrins are not required for the DC migration into afferent lymphatics in the steady state.46) Podoplanin expressed on lymphatic ECs can capture CCL21 (see above) and also binds a lectin-type molecule CLEC-2. Podoplanin’s binding to CLEC-2 promotes the formation of actin-rich protrusions on DCs, which allow the DCs to spread along stromal scaffolds and support DC motility.63) While integrin-binding molecules including ICAM-1 and VCAM-1 are expressed at only low levels in lymphatic ECs, their expression is strongly upregulated during inflammation, and they contribute to DC migration by interacting with β2 integrins (LFA-1 and Mac-1)64) and β1 integrin VLA-4,65) respectively.
Lymphatic ECs produce an immunomodulatory molecule, semaphorin 3A. This molecule promotes actomyosin contraction in the trailing edge of DCs by binding plexin A1 on DCs and may induce the disassembly of adhesive components at the trailing edge as well, thus promoting DC transmigration.66)
Once lymphocytes have entered the lymph nodes, they search for antigen by moving in the cell-dense three-dimensional network in the parenchyma, a highly confined environment. Katakai et al.67) reported that ATX produced by lymph node stromal cells promotes integrin-independent, Rho-dependent interstitial T cell motility in lymph nodes. Our group subsequently reported68) that ATX produced by fibroblastic reticular cells (FRCs) generates LPA through its enzymatic activity and that LPA enhances lymphocyte motility through the densely packed lymph node reticular network (Fig. 3c). Imaging mass spectrometry analysis showed that biologically relevant LPA species (LPA 18:1, 18:2, and 20:4) are expressed in the lymph node paracortex at sites that are either close to or distant from HEVs and that the latter is selectively reduced in mice that are ATX deficient specifically in FRCs,68) in agreement with the hypothesis that FRCs produce LPA by the ATX on their cell surface. Furthermore, intravital two-photon microscopic analysis showed that T cell migration in the parenchyma is significantly attenuated in the conditional ATX-deficient mice compared to control mice and that the ATX/LPA-dependent T cell motility is mediated by the LPA receptor LPA2 on the T cell surface.68) These results, together with the finding that LPA activates Rho-ROCK-myosin II pathways via LPA2 in T cells, suggest that the LPA generated by FRCs acts locally on T cells via LPA2, thereby regulating T cell contractility and motility in the lymph node reticular network68) (Fig. 3c).
Lymphocyte egress from lymphoid tissues is currently thought to be regulated primarily by sphingosine-1-phosphate (S1P), which is structurally similar to LPA. S1P acts on a family of five G-protein-coupled receptors (S1P1–S1P5) and is rapidly degraded into a biologically inactive form by S1P phosphatases and S1P lyase in vivo.
S1P is released from erythrocytes and vascular ECs, causing a high S1P concentration in the peripheral blood. In contrast, S1P is relatively scarce in lymphoid tissues, due to an abundance of S1P lyase. This differential concentration of S1P between the blood and lymphoid tissue is currently thought to drive lymphocyte emigration from lymphoid tissues. Lymphocytes within the lymphoid tissues express high levels of the S1P receptor S1P1 and thus undergo chemotaxis in response to the S1P gradient. In contrast, peripheral blood lymphocytes express low levels of S1P1, due to its downregulation by internalization in response to the high S1P concentration in the blood. When blood-borne lymphocytes enter lymphoid tissues, S1P1 is upregulated due to the paucity of S1P in the tissue. Within the lymph nodes, the lymphocytes are then transported to the cortical sinuses, the medullary sinus, and finally to the efferent lymphatics, by sensing the S1P concentration gradient in an S1P1-dependent manner. This cyclical change in lymphocyte S1P1 expression, which has been proposed to direct lymphocyte egress from the lymph nodes,69) may similarly regulate lymphocyte egress from the thymus.
However, this widely accepted hypothesis cannot be readily reconciled with the following findings. First, S1P1 transcripts are also abundant in the ECs and vascular smooth muscle cells surrounding blood vessels, and strong S1P1 activation is detected in both the lymphatic and vascular ECs in lymphoid tissues, where most lymphocytes show no evidence of S1P1 activation under homeostatic conditions.70) Second, S1P1 is also expressed at high levels on macrophages, DCs, and natural killer cells, but only lymphocytes exit the lymph nodes in response to physiological concentrations of S1P. These findings indicate that S1P’s role in regulating lymphocyte egress is more complex than described so far.
S1P also appears to regulate the barrier function of the HEVs in antigen-stimulated lymph nodes. Herzog et al.71) reported that platelets migrate across HEVs together with lymphocytes in mesenteric lymph nodes and are activated by specific interactions between the platelet cell surface lectin CLEC-2 and podoplanin, expressed on the surrounding FRCs. The activated platelets then secrete S1P, which stimulates the HEVs to maintain their vascular integrity. However, the effect of the podoplanin-CLEC-2 interaction on HEV integrity can only be detected in mesenteric lymph nodes, where exogenous antigens are abundant, and not in peripheral lymph nodes unless the mice are immunized, implying that this mechanism is important under inflammatory conditions.
In the past decade, we have learned many details about the molecular mechanisms underlying immune cell migration across blood and lymphatic vessels. However, many questions and challenges still remain. For instance, we still do not know what promotes the generation of HEVs in tissues. This information is important for devising novel therapeutic approaches for autoimmune diseases and cancer, because by increasing or decreasing HEVs, one should be able to control the lymphocyte recruitment into tissues, and thus the immune status of particular tissues. We also do not know what regulates the permeability of HEVs; for example, whether increased HEV permeability is an intrinsic property of ECs or of other cells surrounding the HEVs, or whether lysophospholipids including LPA and S1P are involved in this phenomenon. This knowledge would also be relevant for the artificial manipulation of immune responses. Given that it is now clear that both blood vascular and lymphatic vascular ECs are the critical regulators of immune cell trafficking, elucidating the above points will bring new insight into the host defense mechanisms and pathogenesis of inflammatory disease, and will provide valuable information for the design of anti-migration therapies for inflammation.

Masayuki Miyasaka was born in 1947 in Ueda, Nagano Prefecture. He is Professor Emeritus of Osaka University, Japan, and a FiDiPro (Finland Distinguished Professor) of the Academy of Finland. He was formerly Professor and Chairman of the Laboratory of Immunodynamics, Department of Immunology and Microbiology at the Osaka University Graduate School of Medicine in Osaka, Japan (1994–2012). He was President of the Japanese Society for Immunology (2006–2008). He was also an Editor of FEBS Letters (1998–2012), an Associate Editor of Immunology (2004–2007), and an Associate Editor of International Immunology (1989–2017). He received the M.D. from the Kyoto University School of Medicine in Japan in 1973 and Ph.D. in immunology from the John Curtin School of Medical Research, Australian National University in Canberra in 1981. He then served as a member of the Basel Institute of Immunology in Switzerland (1981–1986), where he studied the ontogeny of the lymphoid system and lymphocyte migration. Currently, Dr. Miyasaka is interested in the molecular mechanisms underlying lymphocyte trafficking into various tissues and also the mechanism of tumor metastasis in vivo. Main topics of his research are 1) physiological recruitment of lymphocytes and dendritic cells from the body into secondary lymphoid tissues and 2) functions and regulation of intercellular adhesion molecules and chemokines, and their regulators.
We are grateful to Dr. Kazuhito Yamaguchi for allowing us to use his splendid SEM photographs of HEVs. We also thank Prof. Tamio Yamakawa for providing us the opportunity to compile this manuscript. One of the authors (MM) wishes to thank his mentors, Drs. Masahiko Kotani, Peter McCullagh, and Zdenek Trnka for their wisdom, guidance, and support throughout the years. Masahiko Kotani introduced MM to the subject of lymphocyte recirculation and has deeply inspired his research ever since. Peter McCullagh encouraged MM to pursue his research endeavors. His sincere enthusiasm for science sets the standard to which MM seeks to aspire. MM is grateful to Zdenek Trnka for many inspiring conversations and helpful suggestions during his tenure in Basel, Switzerland. The authors acknowledge all the members of the Laboratory of Immunodynamics, the Graduate School of Medicine, Osaka University, and our collaborators, who actively participated in the experiments discussed herein. Because of space limitations, we were able to cite only a portion of the relevant literature, and we apologize to colleagues whose contributions might not be appropriately acknowledged in this review.