2017 Volume 93 Issue 7 Pages 483-497
2017 Volume 93 Issue 7 Pages 483-497
One of the most fundamental questions in neurobiology is how proper synaptic connections are established in the developing brain. Live-cell imaging of the synaptic structure and functional molecules can reveal the time course of synapse formation, molecular dynamics, and functional maturation. Using postsynaptic scaffolding proteins as a marker of synapse development, fluorescence time-lapse imaging revealed rapid formation of individual synapses that occurred within hours and their remodeling in culture preparations. In vivo two-photon excitation microscopy development enabled us to directly measure synapse turnover in living animals. In vivo synapse dynamics were suppressed in the adult rodent brain, but were maintained at a high level during the early postnatal period. This transition in synapse dynamics is biologically important and can be linked to the pathology of juvenile-onset psychiatric diseases. Indeed, the upregulation of synapse dynamics was observed in multiple mouse models of autism spectrum disorders. Fluorescence imaging of synapses provides new information regarding the physiology and pathology of neural circuit construction.
In the vertebrate brain, postmitotic neurons are generated from neuroepithelial cells and they start to extend long axonal processes and relatively short, branched dendrites after their migration to appropriate brain regions. Axonal processes recognize their target postsynaptic dendrites using multiple molecular keys and start to differentiate synaptic structures.1) In the mammalian forebrain, synaptogenesis starts before birth, but the number of synapses significantly increases in the early postnatal period.2) Early studies on synapse formation utilized several types of model synapses in the peripheral nervous system (PNS). One popular preparation is that of neuromuscular junctions (NMJs), which have simple connectivity between presynaptic motor neurons and postsynaptic muscles.3) Another advantage of NMJs is their large size, which facilitates experimental manipulation and structural analyses. Finally, NMJs are accessible by relatively simple operative procedures and can be imaged by conventional fluorescence imaging systems.4) By capitalizing on these advantages, cell and molecular biological analyses of NMJs have greatly advanced our understanding of synaptogenesis in PNS.
In contrast, studies on synapse formation in the central nervous system (CNS) were technically difficult for multiple reasons. First, connectivity of the neurons in CNS is complex, and reliable identification of presynaptic neurons connecting to their target is sometimes impossible because of the lack of appropriate markers.5) Therefore, studies examining CNS synapse formation were mainly conducted in a few specific types of synapses, namely, those in which presynaptic and postsynaptic components are well-defined and both divergence and convergence of axonal projections are low, such as climbing fiber synapses on cerebellar Purkinje cells.6) Second, the synaptic structure in CNS is small, with a typical size of a few micrometers in diameter (Fig. 1).7) Details of the synaptic structure cannot be resolved by conventional light microscopy, and their shape and size could only be measured using electron microscopy (EM). However, three-dimensional reconstruction of EM images requires a large amount of time and labor.7) Recently this situation dramatically changed because of the introduction of new technologies in light and electron microscopy, such as super-resolution microscopy8),9) and focused ion beam/scanning electron microscopy (FIB-SEM).10) Finally, synapses in CNS are difficult to access experimentally.11) In the 1980s, there were no available technologies that would allow access to the small rodent cranium-covered brain without damaging the CNS neurons, which are highly vulnerable to lower oxygen concentrations, increases in intracranial pressure, and inflammation.

Morphology and molecular assembly of the postsynaptic component. (A) EM images of synapses formed in the hippocampal slice culture (left) and dissociated culture of hippocampal neurons. Synaptic vesicles, presynaptic active zone, and PSDs can be recognized. Bars, 200 nm. (B) A scheme of presynaptic and postsynaptic structures. Most of the excitatory glutamatergic synapses on the forebrain pyramidal neurons are formed onto dendritic spines. (C) Glutamate receptors (AMPA receptor, NMDA receptor, and mGluR), postsynaptic scaffolding molecules (PSD-95, guanylate kinase-associated protein (GKAP), Shank, and homer), and postsynaptic adhesion molecules (neuroligin, leucine-rich repeat transmembrane protein (LRRTM), synaptic adhesion-like molecule (SALM), and synCAM) and their organization in PSD. Presynaptic cell adhesion molecules, neurexins, bind to both neuroligins and leucine-rich repeat transmembrane protein LRRTMs. (D) Organization of polymerized actin and actin regulators (Arp2/3 and WAVE complex, formin family proteins, and VASP) in the spine.
To circumvent the difficulty in analyzing CNS synapses, isolated preparations of the nervous system, such as dissociated neuronal cultures, were extensively utilized to identify the nascent synapses formed between CNS neurons.12) The identification of synapses was further facilitated by the development of a culture system of pure hippocampal or cortical neurons without contamination by glial cells.12),13)
A turning point in our ability to detect synapse formation in live samples was reliable marker development for the detection of presynaptic or postsynaptic structures (Fig. 1B). Integral membrane proteins in synaptic vesicles, such as synaptophysin, or proteins associated with synaptic vesicles, such as synapsin I, are good candidates for presynaptic markers. These presynaptic molecules tagged with green fluorescent protein (GFP) and other fluorescent proteins are widely used for the detection of presynaptic components.14),15) A characteristic structure present in the postsynaptic compartment is the postsynaptic density (PSD), a disk-like molecular assembly with a diameter of less than 1 µm that consists of both membrane and cytoplasmic proteins.16),17) Biochemical and proteomic studies identified several hundred protein species present in PSD (Fig. 1C). PSD scaffolding proteins are a small subset of PSD proteins that are proposed to form a framework for the PSD structure.18) They are abundant in the PSD fraction and comprise multiple domains for binding with other postsynaptic molecules.19) A predominant PSD scaffolding protein PSD-95 was originally identified by the biochemical characterization of the purified PSD fraction.20) PSD-95 comprises three PDZ domains, one GK domain, and one SH3 domain. These domains have specific binding partners, including N-methyl-D-aspartic acid (NMDA)-type glutamate receptors,21) synaptic cell adhesion molecules neuroligins,22) and transmembrane α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor regulatory proteins (TARPs).23) Several research groups, including ours, found that PSD-95 tagged with GFP can reliably report the position of PSDs in the dendrites of living cortical and hippocampal neurons.24)–26) This finding greatly facilitated studies on synapse formation in cell biology. By expressing PSD-95-GFP in cultured neurons, the fate of single postsynaptic sites can be followed over several weeks. Live imaging of PSD-95-GFP provided quantitative information about the basic behavior and turnover of the synaptic structure, its regulation by neuronal activity, and its responses to pathological conditions.27)
In the initial studies on CNS synapse development, researchers collected static images of neurons stained with antibodies specific for presynaptic or postsynaptic molecular markers at different time points. These immunocytochemical studies reported gradual increases in the density of presynaptic boutons and postsynaptic PSDs throughout the course of neuronal circuit development. Consistent with data obtained in vitro, immunohistochemical analyses of cortical and hippocampal tissue sections also revealed a gradual increase in synaptic density during postnatal development. These results were taken as evidence for the stability of synapses after initial formation and for their slow differentiation into mature synapses.
Based on the model of immediate stabilization and monotonous accumulation of differentiating synapses, we initially hypothesized that imaging of synapses in live neurons with an interval of several days could detect very few changes in synaptic connections, with only a minor fraction of gained or lost synapses. To our surprise, distribution of PSD-95 puncta at two time points was markedly different, even if the time interval was set to be 24 h (Fig. 2A).26) This observation clearly indicates that formation of individual synapses is a rapid process that takes place over hours. A large proportion of newly formed PSD-95 clusters were already associated with the synaptophysin-positive presynaptic puncta (Fig. 2C). Formation of PSD-95 clusters was synchronized with the formation of dendritic spines (Fig. 2B).15) These observations are consistent with the idea that presynaptic and postsynaptic components are assembled within a narrow time window, and their differentiation is coordinated with one another.28)

Formation of synapses detected by time-lapse imaging in culture preparations.15),26) (A) Time-lapse fluorescence imaging of PSD-95-GFP in a culture of hippocampal neurons. Formation of new PSD-95 clusters can be detected within 12 h (arrows). (B) Formation of PSD-95 clusters (arrows in row b) in newly formed dendritic spines filled with cyan fluorescent protein (CFP, arrows in row a). Merged color images are shown in row c (green: CFP, red: PSD-95- yellow fluorescent protein (YFP)). Times are shown in the right upper corners in minutes. (C) Simultaneous imaging of synaptophysin-CFP (column a) and PSD-95-YFP (column b). Arrows indicate the formation of presynaptic synaptophysin and postsynaptic PSD-95 clusters. Scale bars, 3 µm.
Another surprising observation in the synapse live imaging was a high fraction of lost synapses within a short period. Synapse elimination was high, not only in the early phase of differentiation but also after complete maturation of neurons that had been maintained for more than 3 weeks in culture. The number of gained synapses is balanced with that of lost synapses after the maturation of culture preparations, indicating that the balance between gain and loss of synapses determines the overall trend of synapse density increase. Our initial protocol of expression of fluorescent probes was based on adenovirus-mediated gene transduction.26),29) This technique can achieve high efficiency of gene transduction, but cell toxicity associated with adenovirus infection limited the observation period to less than several days. It is difficult to trace the fate of individual synapses from images taken at short intervals. To achieve long-term observation of the synapse population on the same dendrites, it is necessary to generate a long-term, stable expression system of GFP-tagged synaptic proteins. We generated transgenic mouse lines expressing PSD-95-GFP or another abundant postsynaptic scaffolding protein, Homer1c, tagged with GFP and performed fluorescence time-lapse imaging of cultured neurons taken from these mouse lines.30) Observation of synapse turnover along the same dendrites over weeks provided further information about basic principles of synapse turnover (Fig. 3). The patterns of increase in synaptic density within dendritic segments were variable; half of the dendritic segments showed a monotonous increase in synaptic density (Fig. 3A). Namely, majority of the synapses persist after formation. Interestingly, the other half of dendritic segments showed a complex pattern in changes of synaptic density, with higher chance of synapse elimination within several days after formation (Fig. 3B). The newly formed dendrites rapidly acquired new PSD structures and their density became comparable with that of parental dendrites within a few days. When the fate of single PSD structures was monitored over several days, elimination of newly formed synapses was frequently observed. These data confirmed the importance of the balance between the gain and loss of synapses during neural circuit development and that synapse loss is not confined to a specific subgroup in the total synapse population.

Long-term observation of the same dendrites for turnover of postsynaptic densities.30) (A) Time-lapse images of the dendritic segment showing a monotonous increase in PSD density. The right column shows binarized images of PSDs. (B) Dendritic segments showing a complex pattern in PSD density. DIV; days in vitro. PSDs were detected by Homer1c-GFP. Scale bars, 5 µm.
Dendritic spines are small protrusions from the surface of dendritic shafts. Although dendritic spines are taken as a structural marker of excitatory synapses, a subset of neurons, such as forebrain inhibitory neurons, develop dendrites with few spines and receive most of the excitatory synapses directly onto dendritic shafts.31) This observation clearly indicates that dendritic spines are not essential in basic excitatory synaptic transmission.32),33) The accumulating evidences indicate that spines have modulatory functions in postsynaptic signal transduction and synaptic plasticity.34) Dendritic spines are proposed to be important in multiple aspects of plasticity-related mechanisms, such as confinement of signaling molecules, such as calcium35) and activated small GTPases,36) and attenuation of local membrane depolarization induced by neurotransmitter receptors and voltage-dependent channels within spine heads.37)
Relationship between excitatory postsynaptic sites and dendritic spines is not fixed but regulated along development of pyramidal neurons. In the mature brain, pyramidal neuron dendrites are covered by spines. However, immature pyramidal neurons express highly motile filopodia and lack spines.38) It is likely that a fraction of the dendritic filopodia in an immature stage is converted to spines. However, it has not yet been clarified whether most of spine synapses are indeed generated from filopodia, working as precursors of spines. The filopodia model of spine formation proposes that interactions of motile filopodia with nearby axons initiate synaptic contact development.39) This model is based on light microscopic observations of synapse formation in cultured neurons.15),40) In these experiments, the appearance of dendritic filopodia generally preceded the presynaptic contacts and formation of the PSD structure (Fig. 2A and B). On the other hand, EM-based observations of developing cortical tissue indicated an abundance of shaft synapses in the early stage of cortical development.38) The “Miller-Peters model” was proposed based on this observation and states that direct contact between presynaptic axons and dendritic shafts leads to the formation of synaptic junctions, and this event triggers the subsequent formation of dendritic spines.39) In vitro slice culture data support this model by showing an initial formation of PSD clusters on dendritic shafts and subsequent generation of spines at the same dendritic positions.41) In spite of many previous efforts to identify real precursors of mature dendritic spines, the debate between the filopodia model and Miller-Peters model has not yet been settled.
Recent technical developments in uncaging of neurotransmitters by two-photon excitation lasers have enabled researchers to stimulate cell surface receptors with a high spatial resolution.42) Uncaging of glutamate or GABA on dendritic shafts can induce the formation of new dendritic protrusions, which subsequently acquire synaptic contacts.43),44) The distinction between filopodia in immature dendrites and the dendritic protrusions induced by local neurotransmitter application has not been clarified. Nevertheless, these studies suggest that dendritic protrusive activity triggered by neurotransmitters released from nearby axons contributes to the formation of new synaptic connections.
As described in previous chapters, the turnover of single synapses was mainly investigated in isolated preparations, such as dissociated cultures and organotypic slices, until late 1990s. This situation was dramatically changed by two-photon excitation laser scanning microscopy development and its application in the detection of single synapses in vivo.45) Using this technique, the behavior and fate of single synapses can be followed for hours, days, and months in living animals. Nowadays, in vivo two-photon imaging has become an indispensable imaging technique for investigating synapse development and plasticity; it has greatly contributed to the identification of new mechanisms that underlie neural network development, learning-related formation of new synapses, and synapse pathology related to neurological and psychiatric disorders.
Initial studies on in vivo synapse imaging relied on dendritic spines for the identification of synapses.46),47) As discussed in the previous chapter, reliable detection of multiple types of synapses, in the developing and mature brain, requires other detection methods. Application of PSD-95-GFP as a second marker of postsynaptic specialization was already proven to be useful in culture preparations, and recent studies have demonstrated reliable detection of synapse-containing spines by double imaging of the spine structure and PSD-95 clusters (Fig. 4).48),49) A large fraction of inhibitory synapses are also present on dendritic shafts, and their detection requires specific molecular markers.31) Gephyrin is a major scaffolding molecule of inhibitory postsynaptic specialization. Gephyrin tagged with GFP has been shown to be useful in the detection of inhibitory synapses in live neurons, both in vitro and in vivo.50)–52)

Procedures for in vivo two-photon imaging. (A) Electroporation of plasmids for the expression of PSD-95-GFP and DsRed2 (red fluorescent protein) into the mouse embryos. (B) In vivo two-photon imaging of mice which underwent in utero electroporation of expression plasmids. (C) A single two-photon excitation scanning image taken from the anesthetized mouse. (D) Three-dimensional reconstruction of an image stuck with two fluorescence signals. (E) Enlargement of a dendritic segment marked by a white rectangle in (D). Individual synapses can be reliably detected by the morphology of spines and presence of PSD-95-GFP clusters. In vivo two-photon images are shown as pseudocolor pictures with PSD-95-GFP in green and volume filler DsRed2 in red. Scale bars, 10 µm (C), 3 µm (E).
Application of two-photon microscopy for the visualization of dendritic spines in vivo has been restricted to adult rodents until recently. Early in vivo imaging studies reported discrepancies in the spine turnover rate for transcranial imaging depending on the surgical procedures.46),47) This controversy led to the finding of excess activation of microglial cells due to inappropriate open-skull surgery and microglia-dependent mechanisms of spine pruning.53) Further studies confirmed similar turnover rates of spines obtained by carefully controlled open-skull surgery and by thinning the cranial bone to be transparent enough for spine imaging, indicating that appropriate surgical procedures are necessary for the proper measurement of spine turnover.54) The consensus of researchers working on in vivo two-photon imaging of spines in adult animals is that spine dynamics in the adult cortex is highly suppressed, with a remaining dynamic spine fraction of less than 5% of the total number of spines (Fig. 5).55) As mentioned in previous sections, synapse dynamics in cultured neurons was maintained at a high level, even more than 3 weeks after plating.26) Thus, synapse turnover in vivo is highly suppressed by factors that are not present in reduced preparations of cultured neurons.

Stability of dendritic spines in the adult mouse neocortex. The somatosensory cortex of Thy1-GFP mouse lines were imaged through the thinned skull window with two-photon scanning microscopy.56) The same dendritic segments were imaged at intervals of 1, 14, and 33 days. Most of the spines can be detected on the second imaging session. Scale bar, 3 µm.
There have been few quantitative studies on synapse dynamics in vivo during the early postnatal period. This scarcity of data mainly originates from the difficulty of imaging synapses in the immature brain tissues without activating glial cells. The cranial bone in young pups is thin and less opaque but more vulnerable to invasive techniques. Therefore, the thin skull method cannot be used on mice younger than 2 weeks of age. Our group performed thin skull window-based in vivo two-photon imaging of spines at postnatal 2 and 3 weeks and found extensive gain and loss of both spines and PSD-95 clusters (Fig. 6).56) By comparing images separated by an interval of 2 days, fractions of spine gain and loss were found to be close to 20%. Notably, the turnover rate in vivo in the early postnatal period was comparable with that measured in the cultured hippocampal neurons. This comparison suggests that during early stages of synapse development, spine turnover in in vitro and in vivo preparations share similar mechanisms and that the balance between the gain and loss of synapses determines the profile of synapse density increases during development.

Downregulation of spine synapse dynamics in the postnatal period.56) (A) In vivo imaging of dendrites with the interval of 1 day. In vivo two-photon images are shown as pseudocolor pictures with PSD-95-GFP in green and volume filler DsRed2 in magenta. Arrow indicates a new spine and arrowheads indicate lost spines. Scale bar, 3 µm. (B) Spine turnover rates at different postnatal days. At 8 weeks after birth, spine gain and loss are highly suppressed. (C) Trend of PSD turnover rates during the postnatal period. Note similar suppression of dynamics in both spines (B) and PSDs (C). One-way analysis of variance, Tukey’s test. ***p < 0.001. Number of animals analyzed (2 weeks; n = 6, 3 weeks; n = 6, 8 weeks; n = 3).
Interactions between genetic and environmental factors are critical for the expression of physiological functions of the neural circuits. Construction and refinement of the neural circuits in the neocortex mainly takes place in the postnatal period, and sensory information plays a key role in this process. The developmental profile of spine density in specific brain regions can be used as a readout of the neural circuit construction. In the human neocortex, the peak of spine density is at the age of 2–6 years.57) The rate of spine increase was high before reaching the peak, but the subsequent decline in spine density toward adolescence was very slow. The initial rapid increase of spines and its gradual decrease after the peak is preserved in both monkey58) and mouse neocortex.59) Our quantitative analysis confirmed that spines in mouse layer II/III pyramidal neurons rapidly increase until postnatal week 3.56) The spine population shifts to the phase of gradual decrease thereafter. These observations collectively indicate that an initial phase of rapid construction of neuronal circuits with excess connectivity followed by a phase of slow pruning of unnecessary connections is a common strategy across mammalian species for circuit development and maturation in the neocortex.
The onset of psychiatric diseases is variable, and patients with childhood psychiatric diseases experience symptom onset and diagnosis early in life. Autism spectrum disorders (ASDs) is one of the childhood psychiatric diseases with a high prevalence.60) Patients with ASD show their initial symptoms and can be diagnosed at the ages of 2–6 years. This postnatal period matches the peak of spine density in the human neocortex, suggesting that postnatal neural circuit development is linked to functional changes in the brain of children with ASD.61) Another line of evidence suggests that dysregulated synaptic connectivity has an important role in ASD pathophysiology. Recent genetic studies identified a large number of candidate rare gene variants associated with non-syndromic ASDs.62) Rare variants in genes of postsynaptic scaffolding molecules (Shank2 and Shank3), postsynaptic cell adhesion molecules (neurogilin-3 and -4), and their presynaptic binding partners (neurexin-1) are linked to ASDs.63) These synaptic scaffolding proteins and cell adhesion molecules are critical for the regulation of synaptic connectivity. Therefore, it is likely that dysregulated synapse construction due to genetic changes in key synaptic molecules leads to the accumulation of mismatches in neuronal connectivity, which subsequently triggers symptoms associated with ASDs.
To test this hypothesis of circuit dysfunction in the neocortex of patients with ASD, we applied the technique of in vivo two-photon imaging to multiple ASD mouse models.56) These mouse models include patDp/+,64) NLG R451C,65) and BTBR mice.66) patDp/+ mice mimic the most frequently reported copy number variation in ASD, the duplication of 15q11-13. NLG R451C mice are genetically engineered to contain a rare missense mutation of the Nlgn3 gene, a mutation also found in some individuals with ASDs. The BTBR inbred mouse has been extensively studied and reflect major behavioral features of ASD: deficits in social interactions, unusual vocalization behaviors, and increase in repetitive activities. Because these three ASD mouse models are heterogeneous in their genetic properties, identification of circuit-level impairments shared by these models will lead to the establishment of the working hypothesis for the core pathophysiology of ASDs. Indeed, these three mouse models show consistent upregulation in the turnover of spines positively associated with PSD-95 clusters (Fig. 7). This alteration was specific to the spine synapses receiving intracortical projections; the dynamics of spines receiving inputs from the thalamus were unaffected. Similar upregulation of spine turnover in the neocortex was reported in Fmr1 knockout mice, a fragile X syndrome and syndromic autistic mouse model, supporting the hypothesis of shared synapse-level phenotypes across ASD mouse models with heterogeneous genetic mutations.67),68) patDp/+ and NLG R451C mice further showed impairment in the activity-dependent remodeling of spine synapses in the somatosensory cortex after manipulation of their whiskers and also demonstrated reduced immediately early gene (IEG) responses of layer II/III neurons to the upregulation of whisker-dependent sensory inputs.56) The sensory stimulus-dependent upregulation of IEG was comparable in layer IV neurons between ASD model and control mice, suggesting intact transmission of sensory information from the thalamus to the postsynaptic layer IV neurons. These data indicate that upregulation in spine synapse turnover during the early postnatal period results in weaker functional connectivity within the layer II/III neuron population and/or between layer II/III and layer IV neurons. This dysfunction in information transfer may not be specific to the sensory cortex and may be observed in other neocortical areas including the prefrontal cortex, where circuit impairments can be associated with behavioral deficits in social interactions in patients with ASD.
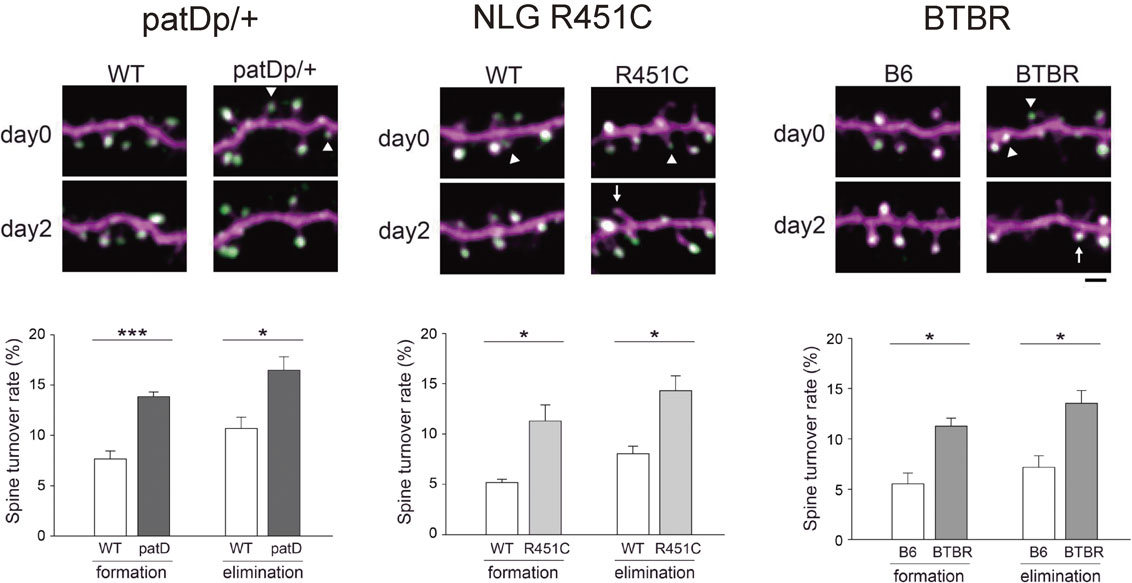
Upregulation of spine synapse dynamics in three ASD mouse models.56) Both formation and elimination rates of spines are upregulated in patDp/+, NLG R451C, and BTBR mouse lines. Arrows and arrowheads indicate spines formed and eliminated, respectively. In vivo two-photon images are shown as pseudocolor pictures with PSD-95-GFP in green and volume filler DsRed2 in magenta. Scale bar, 2 µm. Student’s t-test, *p < 0.05, ***p < 0.001. Number of animals analyzed (patDp/+; control = 5, mutant = 6, NLG R451C; control = 4, mutant = 6, BTBR; C57Bl/6 = 3, BTBR = 3).
An initial phase characterized by the rapid construction of neuronal circuits with excess connectivity followed by a slow synapse pruning phase is a common strategy for neocortical circuit development. In the mature neocortex, turnover of spine synapses is highly suppressed and these synapses can be maintained for several months or even throughout the life of an animal.69)–71) The analysis of synapse stability is as important as that of synapse pruning and plasticity. Without the establishment of stable neuronal connections, long-term storage of information in the brain may be very difficult to achieve. However, there are few studies aiming to clarify underlying principles that support long-term synapse stability. It is possible to propose that unidentified, very stable proteins form the core of PSD and other synaptic molecular interactions are based on the presence of these stable core proteins. Studies investigating molecular mobility in synapses through fluorescence recovery after photobleaching or through photoactivation have provided us with rich information regarding the rate of protein replacement at individual postsynaptic sites. These studies on molecular mobility have failed to find any postsynaptic molecules that stay in the same synapse for a prolonged period.49),72),73) A large fraction of PSD scaffolding molecules are replaced if we monitor FRAP recovery for more than a few hours. The turnover of glutamate receptors, such as AMPA-type and NMDA-type receptors, is in the same range as that of PSD scaffolding proteins.74),75) Therefore, the idea of a stable PSD core complex presently lacks experimental support. Another possibility is the existence of a feedback mechanism for the homeostasis of synaptic molecular assembly and spine volume. Previous studies provided evidence that the actin cytoskeleton supports spine stability.76),77) If a reduction in the actin content in spines activates a feedback loop that triggers the increase of another factor, which then in turn facilitates the recruitment of actin back into the spines, spine actin contents can be maintained automatically. Obvious candidates for actin recruitment factors are regulators of actin polymerization, such as the Arp2/3 and WAVE complexes (Fig. 1D). Indeed, recent super-resolution imaging revealed that PSD can function as a recruitment platform for the WAVE complex.78),79) Previous modeling studies on neutrophil motility proposed the presence of a feedback mechanism between actin assembly and WAVE complex activation.80) In this feedback model, actin is both an output and input to the membrane-tethered WAVE complex, and the spatiotemporal cycling of actin assembly is driven by an active feedback process with the WAVE complex. If a similar feedback mechanism exists in the spine actin meshwork and PSD-tethered WAVE complex, long-term maintenance of spine structure and spine actin organization can be explained. To confirm the presence of this feedback pathway, new technologies that combine mathematical modeling with actin and WAVE complex manipulation in a higher temporal resolution may be required.
Both reduced preparations of neuronal circuits in vitro and recently developed monitoring technology of in vivo synapses are required for an in-depth understanding of the underlying mechanisms of synapse formation, remodeling, and stabilization. The measurement of multiple parameters within single synapses across a large volume of the brain tissue will become important for studies that require comprehensive information regarding the local circuit function associated with specific cortical functions. These imaging experiments will require high speed imaging and an improvement in the spatial resolution of two-photon microscopy.81),82) New technologies that will enable the acquisition of images from wider fields of view that encompass multiple neocortical areas while still maintaining the resolution of single synapses will be required.83) To achieve this goal, it will be necessary to design new microscopic objectives with higher numerical apertures and a wider field of view; additionally, a redesign of optical systems and scanning devices should follow thereafter. Acquisition of data from large volume samples increases the data size and necessitates automatic pipeline development for data analysis, which may require collaboration with data scientists. Finally, the application of synapse imaging techniques to mouse models of psychiatric disorders facilitates the identification of the core pathology in the dysfunctions of neural circuits. This information will be useful for new biomarker development of psychiatric disorders and for the evaluation of new drug candidates and other therapeutic approaches.

Shigeo Okabe was born in Tokyo in 1960 and graduated from The University of Tokyo, School of Medicine in 1986. He was appointed as a research assistant in the Department of Anatomy and Cell Biology, The University of Tokyo in 1988 and obtained Ph.D. degree in 1993 from the Graduate School of Medicine, The University of Tokyo. The main research topic as a graduate student was dynamics of neuronal cytoskeleton. From 1993 to 1996, he worked as a Visiting Associate in National Institute of Neurological Disorders and Stroke, National Institutes of Health and studied neural stem cell biology. In 1996, he came back to Japan and appointed as a principal investigator in National Institute of Bioscience and Human-Technology in Tsukuba. In this new laboratory, he started imaging research in synapse formation and remodeling. In 1999, he moved to Department of Cell Biology, Tokyo Medical and Dental University as a professor. In 2007, he was appointed as a full professor in Department of Cellular Neurobiology, Graduate School of Medicine, The University of Tokyo. His main research interest is dynamic behaviour of synaptic molecules in the process of neural circuit formation. He discovered that dynamic balance between gain and loss of synapses is essential in proper formation and function of cortical neuronal circuits. This finding is important in pathophysiology of neurodevelopmental disorders, such as autism spectrum disorders. He received Young Investigator Award (Japanese Association of Anatomists) in 1996, Tsukahara Nakaakira Award (Japan Neuroscience Society) in 2004, and Seto Award (Japanese Society of Microscopy) in 2010.
The author expresses his gratitude to the collaborators on the study described in this review. This study was supported by grants-in-aid for Scientific Research (26250014 and 25117006), Core Research for Evolutional Science and Technology from the Japanese Science and Technology Agency (JPMJCR14W2), and the Uehara Memorial Foundation.