2019 年 95 巻 6 号 p. 261-277
2019 年 95 巻 6 号 p. 261-277
Vacuolar-type ATPase (V-ATPase), initially identified in yeast and plant vacuoles, pumps protons into the lumen of organelles coupled with ATP hydrolysis. The mammalian counterpart is found ubiquitously in endomembrane organelles and the plasma membrane of specialized cells such as osteoclasts. V-ATPase is also present in unique organelles such as insulin secretory granules, neural synaptic vesicles, and acrosomes of spermatozoa. Consistent with its diverse physiological roles and unique localization, the seven subunits of V-ATPase have 2–4 isoforms that are organelle- or cell-specific. Subunits of the enzyme function in trafficking organelles and vesicles by interacting with small molecule GTPases. During osteoclast differentiation, one of the four isoforms of subunit a, a3, is indispensable for secretory lysosome trafficking to the plasma membrane. Diseases such as osteopetrosis, renal acidosis, and hearing loss are related to V-ATPase isoforms. In addition to its role as an enzyme, V-ATPase has versatile physiological roles in eukaryotic cells.
Communicated by Takao SEKIYA, M.J.A.
Vacuolar-type proton pumping ATPase (V-ATPase), initially identified in Saccharomyces cerevisiae and plant vacuoles, is a ubiquitous enzyme responsible for H+ (proton) transport across membranes and acidification of cellular compartments in animals (see refs. 1–7 for reviews). This enzyme shares its structure and catalytic mechanism with mitochondrial or bacterial F-type ATPase (F-ATPase, ATP synthase), named after Factor of ATP synthesis (coupling factor of oxidative phosphorylation) (see refs. 8–11 for information on F-ATPase). The two enzymes are composed of a membrane extrinsic catalytic sector (F1 or V1) and a membrane intrinsic sector (FO or VO) (Fig. 1) (see refs. 8 and 9 for the similarities between F- and V-ATPases). The subunits of the two enzymes are homologous in structure and function.
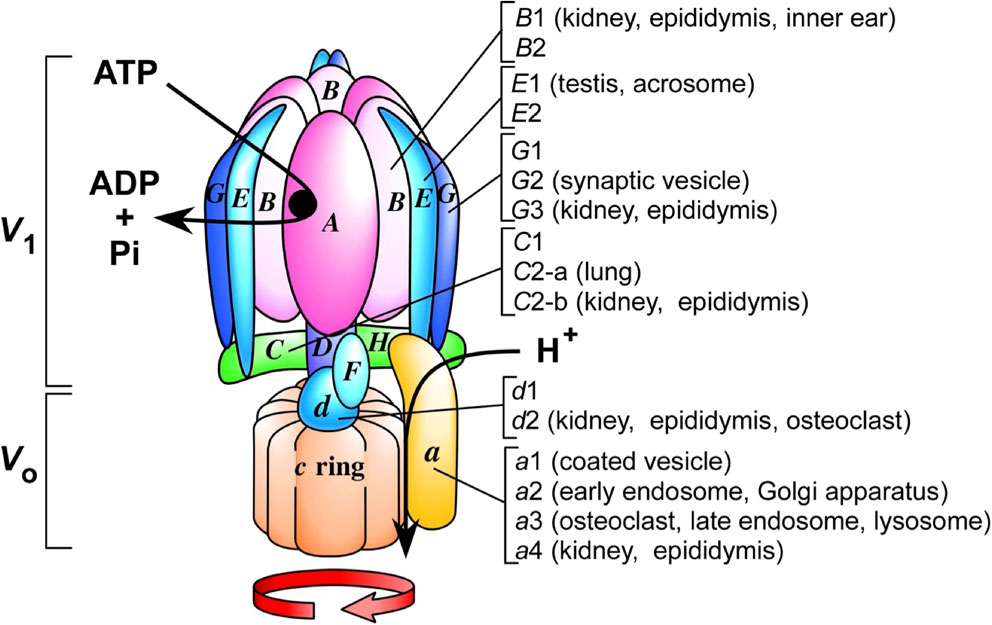
(Color online) A schematic model of a V-ATPase. Model of a proton pumping V-ATPase. A catalytic hexamer (A3B3) in the V1 sector is connected to the transmembrane VO domain through central and peripheral stalks. ATP hydrolysis to ADP and phosphate (Pi) is coupled to rotation of the central stalk, leading to proton transport through a pathway between subunit a and the c ring. Organ-specific isoforms are indicated, and those not specified are ubiquitous. The number of c subunits in the c ring is based on data of cryo-electron microscopic structure of yeast V-ATPase.
Despite these similarities, the two enzymes have different physiological roles except in some bacteria. V-ATPase pumps protons using the energy of ATP hydrolysis, whereas F-ATPase synthesizes most of the ATP using an electrochemical proton gradient. They are different from P-type ATPases (P-ATPases), such as proton-transporting ATPases in the yeast plasma membrane and human gastric membrane (see ref. 12 for a comprehensive book on H+- or cation-transporting ATPases). P-ATPases have a simpler subunit organization and form phosphorylated catalytic intermediates, whereas other ATPases do not.
Similar to F-ATPase, a subunit complex located at the center of the V-ATPase rotates during ATP-dependent proton transport. The phosphate bond energy of ATP is thus converted to a proton gradient across the membrane through the mechanical rotation of subunits. This mechanism, rotational catalysis, was first suggested in extensive biochemical studies and later confirmed through single molecule observation.8)–10)
V-ATPases, which are found in yeast (S. cerevisiae), plants, worm (Caenorhabditis elegans), insects, and mammals, have a high degree of homology1)–7) and are essential for growth, survival, and development. They are targeted to endomembrane organelles including coated vesicles, lysosomes, the Golgi apparatus, secretory vesicles, and the plasma membrane of specialized cells. The proton pumping action of V-ATPases results in the generation of an acidic lumen in various compartments. Consistent with their diverse localization and versatile physiological roles, the membrane extrinsic V1 and transmembrane VO subunits of V-ATPases have many isoforms, which combine to form proton pumping enzymes targeted to different destinations.
The complex structure derived from combinations of isoforms enables V-ATPases to function as more than a simple ATP-dependent proton pump. V-ATPases have active roles in organelle or membrane vesicle trafficking, such as endocytosis and exocytosis, which are essential cellular processes in eukaryotic cells, as well as remodeling of plasma membranes.
Here, we discuss the structure and function of the V-ATPase as a versatile, membrane-bound proton pump, and then focus on its role in organelle or vesicle trafficking, especially in lysosomes. The different functions of V-ATPases are emphasized. For additional topics not discussed in this article or mentioned only briefly, readers can refer to recent reviews.1)–9)
We summarize the properties of the V-ATPase as a proton pumping enzyme in organelles and plasma membranes before discussing its roles in cell biology.
1.1. Unique structure.The basic subunit structure and catalytic mechanism of the V-ATPase are conserved among mammalian and plant enzymes, as summarized in recent reviews.1)–7) The two sectors, V1 and VO, are multimeric subunit complexes with defined stoichiometry. V1 has eight basic subunits (A3, B3, C1, D1, E3, F1, G3, and H1), and VO has six subunits (a1, d1, e, and the c ring, which is composed of three subunits, c, c′, and c′′) (Fig. 1). The numbers indicate the subunit stoichiometry in the enzyme. The catalytic hexamer A3B3, which consists of three of each A and B subunit, hydrolyzes ATP following a cooperative mechanism, as indicated by three Km values for ATP.13) The C, D, F, G, and H subunits are located in the connecting domain between the V1 and VO sectors.
Wilkins and coworkers recently reported the 3.5 Å resolution cryo-electron microscopic structure of the reconstituted VO proton channel of the yeast V-ATPase.14) The authors identified all VO subunits including a, d, e, f, and the c ring. The a subunit has membrane and cytosolic domains that interact with the c ring and V1 subunits, respectively. Biochemical studies identified eight intra membrane helices in the carboxyl terminus.15) A similar structure was obtained using cryo-electron microscopy and evolutional studies.14),16),17) The yeast enzyme contributed to the understanding of the mammalian V-ATPase, as the yeast a subunit has 30–59% sequence identity and approximately 80% similarity with that of humans.4),7)
The c, c′, and c′′ subunits were originally defined as proteolipids or lipoproteins because they are extremely hydrophobic. The three subunits form the c ring, which is similar to the c subunit ring of F-ATPase. In yeast, the c and c′ subunits are encoded by two genes, VMA3 and VMA11, respectively, and are homologous and essential for the functional enzyme.18),19) The c′ subunit is found only in yeast, and the mammalian enzyme has 13 different subunits. The C. elegans c subunit has three isoforms that are expressed at high levels in secretory cells.20)
The cryo-electron microscopic structure shows that the c ring is an assembly of c, c′, and c′′ at a ratio of 8:1:1, and each subunit contains a proton-transporting glutamate residue. Therefore, the c ring is composed of eight, one, and one copies of c, c′, and c′′, respectively, forming the c8c′1c′′1 complex. Protons (H+) are transported through the interface between subunit a and the c ring.14) Because the carboxy terminus of the accessory factor of VO (VOa1), which corresponds to Ac45 in mammals, is an integral membrane component of VO, Ac45 should be considered as a subunit of mammalian V-ATPase. Defining all V-ATPase subunits, particularly those of VO, is difficult because the enzyme interacts with trafficking-related proteins, although the basic subunit structure was described above.
Human V-ATPase genes are designated based on the root ATP6, followed by the sector (V1 or VO), the subunit letter code, and finally the isoform number, if present.21) The genes encoding the A subunit and B1 isoform of the V1 sector are thus defined as ATP6V1A and ATP6V1B1, respectively.
1.2. Proton transport, rotational catalysis, and reversibility.The macrolide antibiotics bafilomycin and concanamycin are specific inhibitors of V-ATPase,13) whereas they have no effect on F- and P-ATPases. The antibiotics bind to the interface between the c ring and the transmembrane helices of the a subunit.22) The antibiotics were used to investigate the mechanism underlying V-ATPase activity, and contributed to our understanding of the physiology of the luminal acidic compartments and the role of the enzyme in intracellular trafficking of vesicles and organelles.
V-ATPase forms an electrochemical proton gradient (pH gradient and membrane potential) that drives active transport into organelles such as yeast or plant vacuoles and mammalian secretory granules. Neurotransmitters such as glutamate and serotonin accumulate inside synaptic vesicles coupled with the generation of a proton gradient or membrane potential.23)–25) A similar mechanism mediates the accumulation of norepinephrine in pituitary microvesicles.26) The accumulation of sugars, amino acids, organic acids, and inorganic ions in plant vacuoles is driven by the generation of a proton gradient.2) Bafilomycin acts as an inhibitor of active transport, as described above.
Similar to the rotational catalysis of isolated F-ATPase or its membrane-bound form,27)–30) ATP hydrolysis drives the rotation of the transmembrane c ring and d subunits attached to the D and F subunits of V1, which are partly inside the catalytic A3B3 hexamer (Fig. 1). Other domains of the enzyme, including the a subunit of VO and A3B3, C, D, E, G, and H of V1, form a stator. Structurally, the central and peripheral stalks are part of the rotor and stator, respectively.
The mechanical rotation of a single V-ATPase molecule was directly observed by introducing a His tag and biotin tag onto the c and G subunits of the S. cerevisiae enzyme, respectively.31) The fluorescent actin probe was attached to the G subunit, and the entire enzyme was immobilized through the c subunit. The c subunit is in the c ring, whereas the G subunit is localized near the A3B3 hexamer (Fig. 1). The addition of ATP triggered the rotation of the actin probe. In this experiment, the rotor including the c subunit was immobilized, and the stator including the G subunit rotated in an ATP hydrolysis-dependent manner. Thus, the rotor and stator are inter-changeable, similar to those in F-ATPase.29),30)
V-ATPase generated essentially the same torque as the F-ATPase.28),30) Rotation was not observed in the presence of concanamycin and nitrate, whereas the F-ATPase inhibitor sodium azide had no effect.31) These results indicated that ATP hydrolysis and H+ transport mediated by V-ATPase involve subunit rotation, as in F-ATPase.
F-ATPase synthesizes ATP coupled with the generation of a proton gradient; however, it can also hydrolyze ATP reversibly to form an electrochemical proton gradient, which is its physiological role in some bacteria.10) Whether V-ATPase, as an ATP-dependent H+ pump, can synthesize ATP is an issue of interest. To investigate the reversibility of the enzyme, the H+-transporting pyrophosphatase of Arabidopsis thaliana, a small flowering plant, was expressed functionally in yeast vacuoles containing endogenous V-ATPase.32) The isolated chimeric vacuoles showed ATP synthesis from ADP and phosphate coupled with the establishment of an electrochemical proton gradient by the plant pyrophosphatase. ATP synthesis exhibited two apparent Km values and was inhibited by bafilomycin. Consistent with this, V-ATPase is homologous with Archaea (Archaea bacteria) ATP synthase (also called A1AO-ATPase),33) suggesting that V-ATPase has the same evolutionary origin with ATP synthase.
These results indicated that V-ATPase is a reversible cooperative enzyme capable of rotational catalysis. However, the same enzyme in endomembrane organelles cannot synthesize ATP under physiological conditions because the enzymes in these organelles cannot generate an electrochemical proton gradient sufficient for ATP synthesis.
1.3. Essential enzyme from yeast to mammals.In contrast to wild-type yeast, the vma mutant (vacuolar membrane ATPase mutant) defective in any one of the V-ATPase subunits does not grow at neutral or slightly alkaline pH.18),19) This mutant can grow at acidic pH, possibly by lowering organelle pH through the incorporation of an acidic medium by pinocytosis (cell drinking). These results suggest that V-ATPase and luminal acidic compartments are essential for yeast growth. This property was used in genetic studies on the yeast enzyme because the mutant phenotype is easy to detect, which contributed to our understanding of the mammalian enzyme.3)
C. elegans is a simple animal model that is useful for the study of bioenergetics. The animal subunits are similar to those of yeast, because the c subunits of yeast and worms share 60% amino acid sequence identity.20) Silencing of the gene encoding the C subunit of V134) or the c subunit of VO20) by RNA interference leads to an embryo lethal phenotype, indicating that the V-ATPase is essential for oocyte maturation and development.
Luminal acidic organelles in mammalian cells can be stained with lipophilic amines such as acridine orange because they are taken up into the acidic lumen, and their protonated forms accumulate in the organelles.35) Bafilomycin renders the staining invisible because V-ATPase is responsible for the acidification. These organelles are present from the one-cell stage of mouse preimplantation embryos.36) The acidic compartments exhibit a polarized perinuclear distribution upon differentiation to trophoblasts at the blastocyst stage. The acidic lumen of organelles can also be visualized by immunoelectron microscopy using a weak base.35)
Mouse embryos in which the c subunit gene is knocked out develop to the blastocyst stage and are implanted in the uterine epithelium;36) however, they are unable to develop further and die shortly thereafter. Because the c subunit is encoded by a single gene, these results indicate that intact V-ATPase and luminal acidic organelles are essential for mouse development.
Although mutant blastocysts attach to the culture dish, the inner cell mass grows at a significantly slower rate and most cells fail to survive. Organelles show no acridine orange staining, and the uptake of fluorescently labeled dextran is significantly decreased. The Golgi complex is swollen and vacuolated, which may be caused by a shift in the ionic equilibrium of the organelle related to the lack of an acidic lumen.36)
A. thaliana haploid cells lacking the A subunit cannot undergo division.37) The male gametophyte lethal phenotype indicates that V-ATPase is essential for haploid gametogenesis.2)
These studies demonstrate that V-ATPase and the presence of a luminal acidic compartment are essential for early development in mammals, worms, and plants. The mechanism by which the enzyme is targeted to specific organelles in eukaryotic cells is an important issue that needs to be addressed.
The V-ATPase subunit isoforms are ubiquitous and cell- or organelle-specific. The diverse localization of the enzyme in organelles is closely related to the isoforms, especially those forming the VO membrane sector.
2.1. Unique localization dependent on subunit a isoforms.Isoforms of the transmembrane a subunit are found in yeast, C. elegans, chicken, mice, cows, and humans.1)–7) Biochemical and structural studies show that subunit a has eight transmembrane helices.14),15) Yeast has two isoforms, Vph1p and Stv1p; the Vph1p-containing enzyme localizes to the vacuolar membrane and is required for the acidification of the central vacuoles, whereas the Stv1p-containing enzyme localizes to the lumen of the Golgi apparatus and pre-vacuolar compartments.12),38) Analysis of hybrid proteins constructed from the two isoforms indicates that the amino-terminal domain determines organelle localization by interacting with Golgi-enriched phosphoinositide.39)
The C. elegans a subunit has four identified isoforms40) that show 43–60% amino acid sequence identity with those of mammalian subunit a and are expressed in different cells. The vha-5 isoform is expressed in H-shaped secretory cells, vha-6 in the intestine, vha-7 in the hypodermis, and unc-32 in nerve cells. Silencing of each encoding gene results in developmental arrest in worms at different stages, indicating that the isoforms play essential roles at specific developmental stages.
Consistent with its diverse roles in different compartments, mammalian V-ATPase has a variety of isoforms. Of the 13 subunits identified in the mouse or human enzyme, seven have two to four isoforms2) (Table 1). The mammalian a subunit of VO has four isoforms (a1, a2, a3, and a4) that show 47–61% sequence identity with each other and high similarity with the corresponding subunit in other vertebrates.41),42)
| Yeast | Mice or Human | |||
|---|---|---|---|---|
| Subunit | Gene | Localization | Tissue/cell | |
| Subunit | Localization | |||
| A | VMA1 | A | Ubiquitous | |
| B | VMA2 | B1 | Kidney, inner ear, lung | |
| B2 | Ubiquitous | |||
| C | VMA5 | C1 | Ubiquitous | |
| C2-a | Lung | |||
| C2-b | Kidney | |||
| D | VMA8 | D | Ubiquitous | |
| E | VMA4 | E1 | Testis (acrosome) | |
| E2 | Ubiquitous | |||
| F | VMA7 | F | Ubiquitous | |
| G | VMA10 | G1 | Ubiquitous | |
| G2 | Neural | |||
| G3 | Kidney, epididymis | |||
| H | VMA13 | H | Ubiquitous | |
| c | VMA3 | c | Ubiquitous | |
| c′ | VMA11 | No homolog | ||
| c′′ | VMA16 | c′′ | Ubiquitous | |
| d | VMA6 | d1 | Ubiquitous | |
| d2 | Kidney, epididymis, osteoclast | |||
| e | VMA21 | e1 | Ubiquitous | |
| e2 | Brain | |||
| a* | Stv1p | pre-vacuole | a1 | Ubiquitous (synaptic vesicles) |
| Vph1p | vacuoles | a2 | Ubiquitous (Golgi, vesicles) | |
| a3 | Ubiquitous (lysosomes, osteoclast) | |||
| a4 | Kidney, inner ear, epididymis | |||
*Stv1p and Vph1p do not correspond to mammalian a1 and a2, respectively. See text for more detail.
V-ATPases containing four subunit a isoforms are found in different organelles, suggesting that the isoforms target the enzyme to a specific location. The isoforms have a large amino-terminal cytosolic domain and eight carboxy-terminal transmembrane helices that may interact with proteins required for trafficking, supporting the role of the isoforms in targeting the enzyme to specific organelles.1),4) Enzymes containing the a1, a2, and a3 isoforms are expressed ubiquitously, although they localize to different organelles in the same cell.41),42)
The a1 isoform localizes to organelles other than lysosomes/late endosomes, or to the Golgi apparatus.43) This isoform is also found in the synaptic vesicle of neurons.44) In nerve terminals, the synaptic vesicle-specific a1 isoform can be relocated to the presynaptic plasma membrane.
The V-ATPase containing the a2 isoform localizes to the Golgi complex.43) Loss of the gene encoding a2 results in abnormal glycosylation of serum and extracellular proteins, confirming that a2 is important for Golgi function.45) Immunostaining with isoform-specific antibodies indicates that a3 is expressed in late endosomes and lysosomes in the fibroblast cell line NIH3T3 and in macrophage-derived RAW264.7 cells.41),43)
The a4 isoform is highly expressed in epithelium with specialized functions.4),42) It was first identified in renal collecting ducts, and its expression is related to the differentiation of this organ during development. Mutation of a4 causes distal tubular acidosis accompanied by hearing loss and enlarged morphology of the inner ear.46),47) In addition to subunit a, the VO subunit d has d1 and d2 isoforms;48),49) d1 is ubiquitous, whereas d2 is specific for osteoclasts.50) The function of subunits a and d in organelle trafficking is discussed below (sections 3 and 4).
2.2. Other isoforms showing unique localization.Isoforms of V1 subunits are found in mammals (Table 1).1)–7) Two isoforms (B1 and B2) of subunit B have been identified in the mammalian V1 sector.4),7) B1 is expressed specifically in the kidney, epididymis, and hair cells of the inner ear, whereas B2 is expressed ubiquitously. Mutations in the human B1 gene (ATP6V1B1) or a4 isoform (ATP6V0A4) cause distal renal tubular acidosis with sensorineural deafness in humans,47),51) suggesting that V-ATPase with B1 and a4 expressed in renal and hair cells is essential for the regulation of acid/base homeostasis and ion transport required for hearing, respectively.4)
Ubiquitous and kidney-specific isoforms of subunits C,48),52) G,48),53),54) and d48),49) are found in mice and in humans (4) (Table 1). The C2, G3, and d2 isoforms are expressed predominantly in the kidney and lung, whereas C1, G1, and d1 are expressed ubiquitously.48)
The C2 isoform shows further diversity (C2-a and C2-b) caused by alternative mRNA splicing,52) and these isoforms show differential expression in the kidney and lung. V-ATPase with C2-a containing a 48 amino acid insertion is expressed in type II alveolar epithelial cells and targeted to lamellar bodies, which are specialized for the storage and secretion of surfactant phospholipids. A luminal acidic pH may be required for packaging phospholipids, surfactant protein processing, or surfactant protein-dependent lipid aggregation in lamellar bodies.55) C2-b is expressed predominantly in the plasma membrane of renal intercalated cells. Immunochemical analyses revealed that V-ATPases with kidney-specific isoforms (B1, G3, d2, a4, and C2-b) and those with expressed ubiquitously B2, C1, d1, and a (a1, a2, or a3) are found in the same renal cells.52)
Mammalian cells contain two E subunit isoforms with 70% sequence identity. E1 is specific for the testis and acrosomes of spermatozoa, whereas E2 is expressed ubiquitously.56),57) E1 is expressed at approximately 3 weeks after birth, corresponding to the start of meiosis, and specifically expressed in spermatid ATPase. Because the a2 isoform is expressed in acrosomes, the enzyme comprising a2/E1 is essential for the establishment of a luminal acidic pH (∼5.3) necessary for activation of an acrosomal protease (acrosin). The E subunit is well conserved, showing approximately 30% amino acid sequence identity between mice and yeast.
The mouse G subunit has three isoforms, namely, G1, G2, and G3, which show 62% sequence identity.48),53),54) G1 is expressed ubiquitously, whereas G2 is expressed in the central nervous system including cortical hippocampal neurons and cerebellar granular cells. G2 localizes to synaptic vesicles, whereas G1 is not detectable in these organelles, indicating that V-ATPase with G2 participates in synaptic vesicle acidification. Loss of G2 leads to G1 subunit upregulation, suggesting that G2 loss is compensated by an increase in G1.54) G3 is expressed in the epididymis. Immune purified V-ATPase with the G1 or G2 isoform shows essentially the same Km value for ATP and maximal velocity, indicating that the G subunits are not involved in catalysis.
2.3. Subunit functions identified from the hybrid enzyme.Mouse genes including those encoding the C, E, and G subunits can complement yeast mutants lacking the corresponding genes; therefore, the roles of V1 subunit isoforms can be studied by analyzing the mouse/yeast hybrid enzyme. The hybrid enzyme containing the mouse isoform C2-a or C2-b is functional in yeast.52) However, its Km values for ATP and proton transport activities are lower than those of the enzyme with C1 or the native yeast C subunit (Vma5p), suggesting that the C2-a and C2-b isoforms are involved in the regulation of ATPases and proton transport. In this regard, the C subunit is located close to the catalytic A3B3 hexamer and subunit a of the proton pathway (Fig. 1), indicating that it may interact with the subunit a isoform, which is essential for targeting V-ATPase to specific organelles and vesicles.
E1 (testis-specific) or E2 (ubiquitous) can form an active enzyme with other yeast subunits.56),57) However, vacuolar membrane vesicles show temperature-sensitive coupling between ATP hydrolysis and proton transport, indicating that the E subunit is essential for energy coupling. The E1/yeast hybrid V-ATPase is defective in proton transport at 37 °C, whereas it reversibly recovers when the incubation temperature is decreased to 30 °C.56) Consistent with the reversible assembly defect, the subunit a epitope of hybrid V-ATPase is exposed to the corresponding antibody at 37 °C, whereas it becomes inaccessible at 30 °C. The domain between Lys26 and Val83 of E1, which contains eight residues that are not conserved between E1 and E2, is responsible for the unique assembly properties of the E1/yeast hybrid enzyme.58)
To extend these observations, we performed alanine-scanning mutagenesis within the amino-terminal α-helical domain between Lys-33 and Lys-83 of the yeast E subunit (Vma4p). The results showed that Glu-44 is one of the key residues for the glucose-dependent assembly/disassembly of VO and V1,59) which is observed in yeast,60) kidney proximal tubule epithelial cells,61) and insects.4) The E subunit, especially its amino-terminal domain, forms the stalk complex with the G subunit (Fig. 1), which should allow both efficient rotational catalysis and assembly of the enzyme in the vacuolar membrane. Different isoform combinations of the human E and G subunits altered their affinities with the C, H, and a subunits.62),63) The altered affinity may contribute to subunit assembly regulation of V-ATPase.
The mouse d1 isoform forms a hybrid enzyme in yeast lacking the corresponding gene (VMA6).48),49) The Km value of this enzyme is similar to that of the native yeast enzyme, whereas it shows significantly higher proton transport activity, suggesting that the d subunit is involved in the coupling of ATP hydrolysis to proton pumping.
2.4. Plasma membrane V-ATPase in specialized cells.Although V-ATPases localize to various endomembrane organelles, they are targeted to the plasma membrane of highly differentiated cells including osteoclasts, kidney intercalated cells, tumor cells, and male tract epithelial cells, which are required for bone metabolism, urine acidification, metastasis, and spermatogenesis, respectively.4),6) V-ATPase also localizes to the apical plasma membrane of salivary gland epithelial cells, suggesting that proton transport affects ion transport in the salivary gland duct.64)
Studies of subunit a isoforms provided clues that helped our understanding of how V-ATPases are targeted to the plasma membrane. The enzymes containing a1, a2, and a3 are found in organelle membranes, whereas those containing a4 localize to the apical and basolateral plasma membranes of cortical α and β intercalated cells, respectively.42) These results suggest that the V-ATPase with the a4 isoform is important for the function of the kidney proton pump in maintaining acid/base homeostasis.
In the epididymis and vas deferens, V-ATPase is located in the apical pole of narrow and clear cells and required for establishing an acidic luminal pH, which is important for sperm maturation and storage in a quiescent state.65) Strong apical staining for subunit isoforms C1, C2, G1, G3, a1, a2, a4, d1, and d2 is observed in narrow and clear cells (epithelial cells) of the rat epididymis and vas deferens, suggesting that these cells have an a4-containing V-ATPase possibly in the plasma membrane. However, a4 is not expressed in the plasma membrane of osteoclasts,41),43) which prompted us to study the plasma membrane V-ATPase in osteoclasts and other cells.
Bone resorption lacunae are interesting acidic luminal compartments outside the plasma membrane. V-ATPase targeting to the plasma membrane and its roles are discussed in this section.
3.1. Bone homeostasis and V-ATPase.Bone homeostasis is maintained through the equilibrium between bone-genesis by osteoblasts and resorption by osteoclasts. Changes of the equilibrium in either direction cause a pathological state. Decreased resorption causes osteopetrosis, whereas its increase leads to osteoporosis.4),5) Resorption requires an extracellular acidic compartment, bone resorption lacunae formed between the osteoclast plasma membrane and the bone surface.
The acidic pH in resorption lacunae originates from protons generated in the osteoclast cytoplasm by carbonic anhydrase II (CO2 + H2O $ \leftrightarrows $ HCO3− + H+),66) as shown in an osteoclast model (Fig. 2), and these protons are secreted into lacunae by the plasma membrane V-ATPase.4)
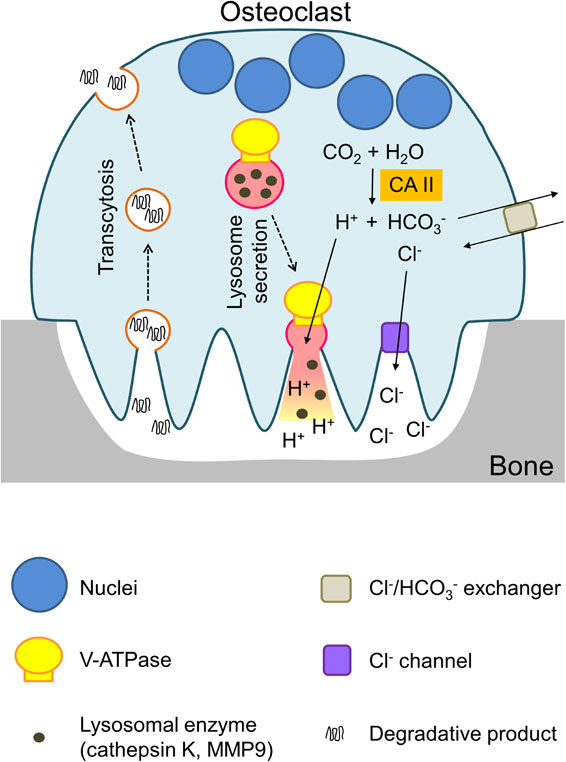
(Color online) Model of osteoclast attachment to the bone surface. Osteoclast attachment to the bone surface is shown together with resorption lacunae. Protons (H+) generated with carbonic anhydrase II (CAII) are secreted by the V-ATPase, and other ions (HCO3−, Cl−) are transported by antiporters and channels, respectively. Bone materials are resorbed from resorption lacunae and transported to the basolateral plasma membranes.
Mutations in a3 increase bone density and cause osteopetrosis.67),68) Because V-ATPase is inactive in the mutant, bone materials are not digested for absorption into osteoclasts. Other transporters are involved in maintaining ion homeostasis in the cytoplasm. For example, bicarbonate (HCO3−) is transported to the outside by the Cl−/HCO3− antiporter, and Cl− ion taken up by the antiporter is secreted to the lacunae by the Cl− channel. The loss of the channels causes diseases including osteopetrosis67),68) and neurodegeneration.69) Lysosomal enzymes secreted together with HCl mobilize the bone matrix mainly composed of collagen and calcium phosphate.
Osteoclasts facing the bone surface are identified as multinuclear cells stained histochemically with tartrate-resistant acid phosphatase (TRAP) (Fig. 3).41),43) Of the four isoforms, a3 localizes to the plasma membrane of the same cell, suggesting that the V-ATPase with a3 is responsible for proton secretion.
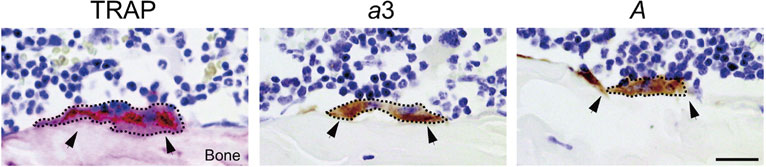
Localization of osteoclasts on the bone surface. Sections of mouse femora were stained histochemically with tartrate-resistant acid phosphatase (TRAP), and immunochemically with antisera against the a3 isoform (a3) and subunit A (A). Multinuclear osteoclasts are surrounded with dashed lines and indicated by arrowheads. Cited from Toyomura et al.43)
Targeting diverse V-ATPases is a cell- and compartment-specific dynamic process. The localization of the a3-containing enzyme to lysosomes of the progenitor suggests that lysosomes are transported to the cell periphery and fused with the plasma membrane. V-ATPase relocated to the plasma membrane can thus secrete protons into the bone resorption lacunae. This model was tested by analyzing lysosome trafficking in the osteoclast cytoplasm. However, osteoclasts are highly specialized multinuclear cells, and establishing an osteoclast cell line is difficult. We analyzed the dynamics of lysosomes during osteoclast differentiation.41),43)
3.2. Differentiation into osteoclasts.Murine macrophage RAW264.7 cells can differentiate into osteoclast-like multinuclear cells upon stimulation with solubilized Receptor Activator of Nuclear Factor kappa B Ligand (RANKL) isolated from osteoblasts.43) The differentiated cells express osteoclast-specific enzymes such as TRAP and cathepsin K, indicating that this cell line is a good model to study differentiation from progenitor to osteoclast. Bone marrow or spleen macrophages can be induced to differentiate into multinuclear osteoclasts using the same protocol established for RAW264.7 cells.43)
Before differentiation, RAW264.7 cells express a3-containing V-ATPase in lysosomes similar to fibroblast-derived NIH3T3 cells.43) Enzymes containing the a1 and a2 isoforms localize to organelles other than lysosomes. We investigated the mechanism underlying lysosome/endosome targeting during differentiation of RAW264.7 cells into osteoclasts. Stimulation with RANKL increased the a3 content with kinetics similar to those of the formation of multinuclear TRAP-positive cells, whereas a1 and a2 did not increase and a4 was not expressed.43) The V-ATPase with a3 colocalizes with lysosome markers including LAMP2 and CD68, as detected by immunochemistry. Genetic defects of a3 increase bone density and cause severe osteopetrosis.67),68)
The d2 isoform of the d subunit connecting V1 and VO was induced and formed a V-ATPase with a3.50) Ubiquitously expressed d1 was not upregulated, and was detected in perinuclear organelles. The d2 isoform is kidney- and epididymis-specific and is not expressed in RAW264.7 cells before induction. The expression of d2 in osteoclasts is 4-fold higher than that of ubiquitous d1, suggesting that V-ATPases with the a3 and d2 isoforms function as major proton pumps in osteoclasts.50) Similar results were obtained with osteoclasts differentiated from mouse spleen macrophages.50)
The findings described above suggest that V-ATPases containing the a3 and d2 isoforms are targeted to the cell periphery during differentiation and incorporated into the plasma membrane of mature osteoclasts, as demonstrated in RAW264.7-derived osteoclasts attached to the mouse skull piece.43) Osteoclast lysosomes, therefore, belong to the family of secretory lysosomes, of which transport to the plasma membrane is induced by the osteoclast-specific ligand RANKL.
RAW264.7 cells form multinuclear cells upon stimulation with lipopolysaccharide, an outer membrane component of Gram-negative bacteria.70) However, these cells do not express TRAP, and lysosome targeting to the plasma membrane is not observed (ref. 70 and M. Nakanishi-Matsui, unpublished observation), indicating that osteoclast formation in these cells is mediated by a different mechanism from that in multinuclear cells.
3.3. Secretory lysosomes require a3 and d2.Because V-ATPases with a3 localize to lysosomes and the plasma membrane of progenitors and osteoclasts, respectively, we address the role of a3 in targeting the enzyme to specific compartments. The plasma membrane of knockout mutant osteoclasts forms a characteristic finger-like membrane fold (ruffled border) facing bone resorption lacunae similar to the wild-type (Fig. 4), indicating that a3 is not essential for the formation of bone resorption lacunae.71) In the mutant, lysosomal marker protein, CD68, was not detected near the cell periphery or plasma membrane, whereas the wild-type clearly showed it in the ruffled border and plasma membrane (Fig. 4A and B). These results suggest that lysosomes are not targeted to the plasma membrane in a3 mutant mice.
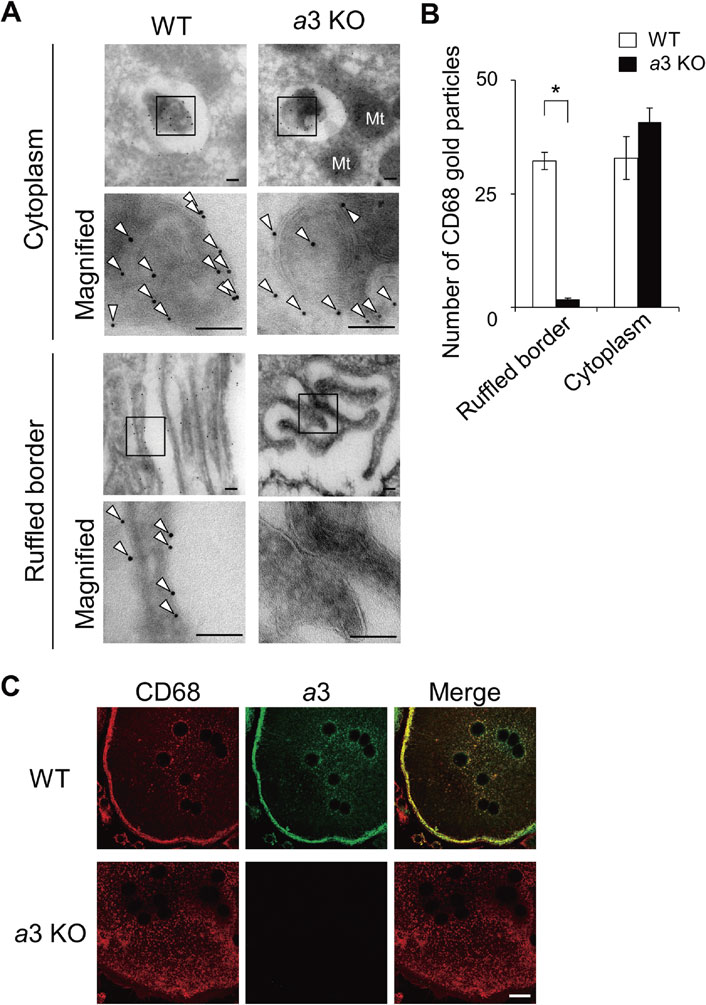
Localization of a lysosomal protein in wild-type and a3-knockout osteoclasts. (A) Histochemical localization of a lysosomal protein (CD68) in wild-type (WT) and a3-knockout (a3KO) osteoclasts. Magnified images of the ruffled border and cytoplasm are shown. The boxed regions were further magnified for immunogold detection (Magnified). Arrowheads indicate labeling of CD68 with 10 nm gold particles. Bars indicate 100 nm. Particles were rarely observed in mitochondria and nuclei. Cited from Matsumoto et al.71) (B) Intracellular distribution of CD68. The numbers of 10 nm gold particles for CD68 were counted in 30 randomly selected fields (42 µm2) of the ruffled border and cytoplasm. Open and closed bars indicate wild-type and a3-knockout osteoclasts, respectively. The mean numbers of particles observed per field are shown with s.e.m.; *p < 0.0001 (unpaired two-tailed Student’s t-test). Few particles were found in the ruffled border of mutant osteoclasts. See ref. 71 for more detail. (C) Localization of a lysosomal protein (CD68, red) and a3 (green) in osteoclasts induced from wild-type (WT) and a3-knockout (a3KO) mouse macrophages with stimulation of RANKL. Merged images are also shown. Bar indicates 20 µm.
Consistent with the in situ observations, macrophages from a3 knockout differentiate into multinuclear cells after stimulation (Fig. 4C).71) These cells are formed following almost the same kinetics as those of wild-type macrophages and contain osteoclast-specific enzymes such as TRAP. Furthermore, the number of TRAP-positive multinuclear cells is comparable between mutant and wild-type macrophages. CD68, a lysosomal marker protein, and a3 well co-localized in the cell peripheral region in wild-type osteoclasts (Fig. 4C, WT), suggesting that osteoclast secretory lysosomes have V-ATPase with a3. However, mutant lysosomes were dispersed in the cytoplasm of multinuclear cells (Fig. 4C, a3 KO) similar to their distribution in cells before stimulation. This indicates that the lysosomes of the mutant are not targeted to the plasma membrane and its vicinity, and the a3 isoform is therefore indispensable for lysosome trafficking to the plasma membrane.
Similar to a3-knockout cells,71) inactivation of the gene encoding the osteoclast-specific d2 isoform results in inefficient cell fusion and the formation of smaller osteoclasts. These cells are defective in proton pumping to bone resorption lacunae, and form an abnormally increased bone mass.72) In a3-knockout cells, the osteoclast-specific d2 isoform is induced and incorporated into the V-ATPase with the a1 or a2 isoform. However, V-ATPases containing the d2 and a1 isoform or those containing d2 and a2 are not targeted to the plasma membrane. These findings indicate that differentiated multinuclear cells do not secrete protons, unlike wild-type cells expressing the enzyme with d2 and a3 in the plasma membrane.
3.4. Role of Rab GTPases and a3 in lysosome trafficking.To understand how the a3 isoform regulates secretory lysosome trafficking in osteoclasts, we focused on the interaction between a3 and Rab family small molecule GTPases, which form a complex with effector and motor proteins, connecting the organelles to cytoskeleton components such as microtubules and actin filaments.73),74) Their activities are regulated by a bound guanine nucleotide: GTP- and GDP-bound forms are active and inactive, respectively. In the inactive state, two prenyl groups covalently attached to the Rab protein interact with a GDP dissociation inhibitor (GDI). After dissociation from the GDI, the Rab protein is activated by a GDP/GTP exchange factor (GEF) and stably localizes to the organelle by anchoring the prenyl groups to the membrane.
Among more than 60 mammalian Rab proteins, Rab775) and Rab27A76),77) are thought to be involved in secretory lysosome trafficking. Downregulation of these Rab proteins results in impaired bone resorption, which may inhibit lysosome targeting to the plasma membrane.
Expression of the dominant-negative form revealed that Rab7, but not Rab27A, is required for trafficking of secretory lysosomes to the plasma membrane. Rab7 is targeted to lysosomes in wild-type osteoclasts, whereas it is distributed in a diffuse pattern in the a3-knockout cell cytoplasm.71) The GTP-bound active form of Rab7 localizes to lysosomes in an a3-dependent manner, which is important for lysosome trafficking. Direct interaction between a3 and GDP-bound Rab7 is highly specific: the GTP-bound form interacts with a3, but only weakly with a1 or a2. As shown in Fig. 5, Rab7(GDP) binds to the a3 isoform of the V-ATPase in the lysosomal membrane, and GDP is then replaced by GTP. The lysosome binds to motor proteins through Rab7(GTP), and the entire complex binds to microtubules. Lysosomes localized to microtubules in osteoclasts are dispersed in the presence of nocodazole, which depolymerizes microtubules.39),43) Supporting this model, Rab7 downregulation impairs osteoclast polarization and thus bone resorption.75)

(Color online) Model of secretory lysosome targeting to the osteoclast plasma membrane. (A) Transport of secretory lysosomes to the plasma membrane. The V-ATPase with a3 located in secretory lysosomes recruits Rab7, and lysosomes are carried to the plasma membrane through microtubules. Rab7(GDP) binds to a3 in the V-ATPase localized in the lysosome membrane, and GDP is replaced by GTP. The lysosome binds to motor proteins through Rab7(GTP), and the entire complex then binds to microtubules and is targeted to the apical plasma membrane of osteoclasts. Cited from Matsumoto et al.71) (B) Secretory lysosomes attached to the plasma membrane. At the final stage, the lysosome binds to the plasma membrane through Rab27. Rab27A(GDP) binds to a3 in the ATPase localized in lysosomes; GDP is replaced by GTP, and the enzyme is tethered to the plasma membrane. Lysosomes containing the two complexes, V-ATPase a3/Rab7 and V-ATPase a3/Rab27, are carried to the plasma membrane.
The a3 isoform in the lysosome interacts specifically with the GDP-bound form of Rab27A (Fig. 5A and B). Lysosomes also function as a secretory compartment in specialized cells, such as the secretion of perforin and melanin from T lymphocytes and melanocytes, respectively. In this regard, Rab27A is involved in a later step of lysosome secretion in cytotoxic T lymphocytes by tethering between secretory lysosomes and the plasma membrane.76),77) These results suggest that the secretory lysosomes complexed with Rab27A through the a3 isoform are tethered to the plasma membrane before fusing with the plasma membrane (Fig. 5B).
These findings revealed an unexpected functional link between the V-ATPase a3 isoform and the Rab7 or Rab27A GTPase, which will contribute to our understanding of the mechanism underlying secretory lysosome trafficking.
Similar to the role of a3 in secretory lysosomes, unique isoforms function in membrane trafficking such as exocytosis and endocytosis.
4.1. Role of V-ATPases in exocytosis.During differentiation to osteoclasts, secretory lysosomes are targeted to the cell periphery and remodel the plasma membrane by incorporating V-ATPases with a3 and d2, concomitant with the secretion of lysosomal enzymes to resorption lacunae. The mechanism underlying the specific exocytosis of lysosomes is dependent on V-ATPase and Rab proteins, suggesting that the a3 isoform plays a regulatory role in lysosome trafficking.
The a3 isoform is highly expressed in pancreatic Langerhans islets, and approximately 80% of the isoform localizes to insulin-containing secretory granules of pancreatic β-cell.78) Mutant mice (oc/oc) with deletion of part of the a3 gene show low blood insulin levels even after administration of glucose. However, the mutant secretory granules contain mature insulin, and the ratio of mature to pro-insulin is similar to that of the wild-type, indicating that the processing is normal in the mutant. Mutant granules from isolated islets show defects in secretion in response to glucose or depolarizing stimulation. Confirming the mutant result, treatment with the V-ATPase inhibitor bafilomycin had no effect on insulin secretion from wild-type islets. These results indicated that a3 is indispensable for the exocytosis of secretory granules, but not for the generation of the acidic lumen of the granule.
During osteoclast differentiation, secretory lysosomes have V-ATPases with the a3 and d2 isoforms. However, lysosomal proteins do not localize in insulin secretory granules, and insulin is not detected in lysosomes.76) This suggests that lysosomal proteins and V-ATPase with the a3 isoform are sorted differently to the two organelles through an unknown mechanism.
Similar to insulin secretory granules, the a3 isoform is strongly expressed in all endocrine tissues tested, including the adrenal, parathyroid, thyroid, and pituitary glands,79) suggesting that the a3 isoform is commonly involved in trafficking during regulated secretion from endocrine cells. However, the a3 isoform is not the only subunit that localizes to secretory vesicles. In nerve terminals, V-ATPase with the a1 isoform is specifically delivered to synaptic vesicles, from which it is relocated to the presynaptic plasma membrane.44),80) Mutation of neural a1 results in the loss of synaptic vesicle exocytosis in Drosophila.44) Analysis of the mutants suggests an acidification-independent role of subunit a1.
Isoforms of other subunits may regulate exocytosis, as suggested by their expression in vesicles specific for secretion or plasma membrane. As discussed previously, the G2 isoform, one of the three isoforms of the G subunit,53) is expressed in the central nervous system including synaptic vesicles, and also regulates exocytosis. Unlike the case of the a3 subunit, suppression of G2 function does not lead to a neurological phenotype. Loss of this isoform upregulates G1 expression in the brain,54) suggesting that G1 compensates, at least partly, for the role of G2.
V-ATPases with C2-a localize specifically to lamellar bodies of type II alveolar cells.52) These organelles have an acidic internal pH of 5.5 and are specialized for the storage and secretion of surfactant phospholipids. The luminal acidic pH and C2-a may be required for packaging surfactant phospholipids, processing of surfactant proteins, or surfactant protein-dependent lipid aggregation.55) As shown in Fig. 1, C2-a localizes to the peripheral stalk and interacts with the A3B3 hexamer and subunit a, suggesting its indirect role in trafficking.
Mouse56) and human57) subunit E has E1 and E2 isoforms, which exhibit more than 70% identity with each other and approximately 30% identity to the yeast E subunit (Vma4p). Mutations of yeast Asp (Asp139) corresponding to mouse Asp145 affect the stability of Vma4p. E2 is ubiquitously expressed in all tissues examined. V-ATPases with the E1 and a2 isoforms are located specifically in developing acrosomes of spermatids and acrosomes in mature sperm. During fertilization, the acrosome membrane fuses with the plasma membrane of sperm, exposing the content responsible for breaking through the coating of the egg and allowing fertilization to occur. This process, known as the acrosome reaction, is similar to exocytosis, and the subunit isoforms E1 and a2 may contribute to the reaction.
4.2. Role of V-ATPases in endocytosis.In contrast to secretory lysosomes or exocytosis, in the endocytic pathway, foreign materials are taken up from the cell surface through the plasma membrane and transported to endosomes/lysosomes. A luminal acidic pH is required for processing toxins, releasing Fe3+ ions from transferrin, releasing cholesterol from low density lipoprotein, and the hydrolysis of foreign proteins including toxins.4) The acidic pH is generated by the V-ATPase, and its inhibition leads to impaired endocytosis. Classic examples of the roles of V-ATPases in endocytosis are the uptake of epidermal growth factor and diphtheria toxin.35),81) Mutant cells lacking the c subunit gene show defective endocytosis of fluorescently labeled dextran, which mostly remains at the cell surface and is not targeted to lysosomes.36)
Similar to exocytosis, the role of the V-ATPase in endocytosis goes beyond generating acidic lumens. The V1 sector H subunit binds to the adaptor protein (AP2) complex,82) which is involved in the internalization of proteins through the plasma membrane to early endosomes.83) Vma13p, the yeast counterpart of subunit H, is required for functional coupling of the V-ATPase.84)
Kidney epithelial cells in the proximal tubule play important roles in protein homeostasis. Proteins such as albumin, hormones, chemokines, and vitamin-binding proteins are reabsorbed and transported to lysosomes for recycling.4) The uptake of proteins for degradation is dependent on the acidic lumen of the endosome. The GDP/GTP exchanger factor ARNO (ADP-ribosylation factor nucleotide-binding site opener) and its cognate Arf6 (small GTPase ADP-ribosylation factor 6) are recruited from the cytosol to the early endosomal membrane in a manner dependent on luminal acidification by V-ATPases.85) Defects in this process lead to proximal tubulopathies in humans and mice.86)
Of four subunit a isoforms, a2 is targeted to the early endosome membrane of the proximal tubule, whereas the a4 isoform is targeted to the plasma membrane.4) ARNO directly interacts with the cytoplasmic domain of the a2 isoform, whereas Arf6 specifically interacts with the c subunit of the enzyme in the early endosome.86) Arf family small GTPases are regulatory proteins involved in vesicular trafficking and organelle biogenesis. The interaction with a2 is pH-dependent, and crucial for protein trafficking between early and late endosomes. The disruption of this interaction by bafilomycin results in the reversible inhibition of endocytosis due to the defective protein trafficking. These studies indicate that V-ATPases modulate vesicular trafficking by recruiting small GTPases, similar to secretory lysosomes.71) Further studies on the downstream targets of the V-ATPase/ARNO/Arf6 complex should be of interest.
V-ATPases transport protons across the membranes of cytoplasmic or extracellular compartments, thereby generating acidic lumens. The similarities to F-ATPases (ATP synthase) contributed to our understanding of the V-ATPase as an ATP-dependent proton pump. However, the two enzymes may function through different mechanisms, since V1 and VO have more complex subunit structures than F1 and FO. Furthermore, V-ATPases interact with other regulatory proteins and play roles in organelle trafficking. Our research on proton pumping ATPases spanning more than three decades was discussed in a previous review.87)
The mechanisms underlying targeting of the enzyme to specific organelles are discussed based on the cell-specific roles of the subunit isoforms. Four isoforms of subunit a are crucial for V-ATPase trafficking to diverse organelles. Other subunits may affect the structure of the a subunit, and are thus involved in trafficking. This possibility is discussed above. During osteoclast differentiation, V-ATPases with the a3 and d2 isoforms are indispensable for targeting lysosomes to the plasma membrane. The a3 isoform is located in secretory vesicles of endocrine cells, whereas a1 and a2 localize to different organelles.
The studies described above raise basic questions in cell biology. One of them is the sorting and targeting mechanisms of different V-ATPases, especially those with a1, a2, a3, and a4, to diverse compartments, namely, how V-ATPases find their destination. Although the mechanism underlying the localization of V-ATPases to the plasma membrane is discussed, it remains unclear how the enzyme recognizes different plasma membranes in an isoform-dependent manner, or how the same V-ATPase is targeted to different organelles, such as the targeting of a3-containing V-ATPases to lysosomes and hormone secretory granules. Another issue that needs to be clarified is how specific lysosome trafficking is induced by specific ligands such as RANKL.
Further investigation of the versatile roles of V-ATPases in diverse compartments should contribute to our understanding of organelle dynamics in the cytoplasm and plasma membranes.
We are grateful to all the co-workers whose contributions were cited in the text and References. This work was supported partly by the grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP08307018, JP20370048 (to M.F.), JP15K07939, JP18K06661 (to M.N.-M.) and JP16K07357 (to N.M.).

Masamitsu Futai completed his graduate studies at the Faculty of Pharmaceutical Sciences, the University of Tokyo, in 1969, and then worked as a postdoctoral fellow at the University of Wisconsin Department of Oncology and Cornell University Section of Biological Sciences, where he worked in the fields of biochemistry and molecular biology. Positions held are as follows: 1977–1985, Professor, Department of Microbiology, Okayama University Faculty of Pharmaceutical Sciences; 1985–2003, Professor, Department of Biological Sciences, Institute of Scientific and Industrial Research; 2003–2006, Special Research Scientist, Microbial Chemistry Research Center; and 2006–2013, Professor and Dean, Iwate Medical University. He has contributed to the field of biological energetics, especially ATP synthesis, proton pumping V-ATPases in organelles and plasma membrane, and gastric proton pump P-type ATPases. Honors received include the Japan Academy Prize and Prize of the Japanese Pharmaceutical Society, and the Fujihara Award.