2019 Volume 95 Issue 7 Pages 321-342
2019 Volume 95 Issue 7 Pages 321-342
New superconductors discovered in the Akimitsu laboratory are reviewed here. These materials can be categorized into two groups:
1) Cu-oxide superconductors.
1-1 Cu-oxide system having CuO2 planes.
1-2 Ladder lattice superconductor.
2) Exploration of new metal-based superconductors.
2-1 MgB2 and its application.
2-2 Y2C3.
2-3 Carrier-doped wide-gap semiconductors.
2-4 New superconductor with a cage-type structure: R5T6Sn18 (R = Sc, Y, Lu; T = Rh, Ir).
Finally, all of the new superconductors discovered in our laboratory are summarized. The outlook for the high-Tc superconductors and our present work are also described.
Communicated by Sumio IIJIMA, M.J.A.
Superconductivity was discovered in mercury (Hg) by Kamerlingh Onnes of Leiden University in 1911 (Tc = 4.2 K). That was three years after the great achievement of helium (He) liquefaction.
The three major characteristics of superconductivity are first pointed out, and then each of them is explained briefly. They are:
It is well known that many free electrons exist in metals, and that an electric current is a flow of those electrons. In general, electrons are scattered by the oscillations of atoms (phonons), or by impurities in metals. This can be described by Ohm’s law. Since all metal contains impurities, and atoms oscillate, electrons are always being scattered. Thus, it seems to be impossible to ever experience zero electrical resistance. However, zero electrical resistance actually exists under superconductivity. It can thus be concluded that if an electric current is initiated in a circular superconductor, it would continue to flow indefinitely. This is a totally mysterious phenomenon and it is a puzzle that motivates many physicists to understand. Its final solution was offered by three physicists, (Bardeen, Cooper and Shrieffer), which is now known as the BCS theory.
There may be many applications of the phenomenon of zero electrical resistance, such as superconducting magnets, superconducting power transmission, and electricity storage. For example, there is a grand plan to set up global networks of electric power plants using wind-power generation, or solar batteries in deserts or waste land, and to send electricity generated in these plants to remote urban areas through superconducting cables.
Another example is linear-motor cars that run by ‘floating their bodies’, using the repulsive power of magnetic fields generated by superconducting magnets.
(2) The Meissner effect.In addition to zero electrical resistance, superconductivity has another unique characteristic. Superconductors have no internal magnetic flux or no lines of magnetic force within their substance (B = 0). This phenomenon is called the Meissner effect, named after its discoverer, which may have many applications, such as magnetic shielding, magnetic levitation, magnetic bearings, and so on.
(3) The Josephson effect.The third major characteristic of superconductivity is the Josephson effect. This is a phenomenon where superconducting electrons flow through between two superconductors separated by a thin barrier, without any voltage being applied. It is a quite unique phenomenon, causing superconducting electrons to flow without a voltage to drive them (DC Josephson effect) or AC current to flow when a DC voltage is applied (AC Josephson effect). In 1962, Josephson, at that time a graduate student at Cambridge University, predicted this phenomenon, specific to superconductivity, that would be a macroscopic effect of quantum mechanics. The most significant application is in a superconducting quantum interference device (SQUID). This may be used in magnetic-field sensors or super high-speed computing machines in the future.
Thus, superconductivity is not only wide and deep as fundamental aspect of physics, but also extremely important concerning applications, and it is expected to be a key to such issues as solving ‘Energy problem’. It is no exaggeration to say The 21st century is a century of superconductivity.
As mentioned above, superconductivity is a very interesting study area, and has many possible applications. The most serious difficulty, however, is that the superconducting state is only achieved below a certain temperature, the critical temperature (Tc), which is usually very low. Nb3Ge was a substance with the highest critical temperature obtained by 1980, at 22 K. B.T. Matthias wrote that a room-temperature superconductor is only pure science fiction. However, many scientists have continuously attempted to find superconductors with a higher Tc, and I was one of those people.
The next problem concerns what guiding principles are required to produce a higher Tc? The only clue is an equation for the superconducting transition temperature based on the BCS theory. According to the simplest BCS theory, the superconducting transition temperature (Tc) can be expressed as follows:
| \begin{equation} T_{\text{c}} \cong \Theta_{\text{D}}\,\exp\left(-\frac{1}{\lambda}\right), \end{equation} | [1] |
| \begin{equation} \lambda = N(0)\cdot V, \end{equation} | [2] |
The parameters that may be easily controlled are the Debye temperature, Θ, and N(0). Matthias focused on substances having a high N(0) (the Matthias Rule). Upon systemic exploration of substances by this method, however, a breakthrough concerning the upper limit of Tc could not be obtained. Therefore, we focused on substances with a large electron-phonon interaction, V. However, when the electron-phonon interaction is increased steadily, electrons are combined in real space, form pairs in real space and stop moving, which becoming insulators. These are called charge density wave (CDW) type insulators. Figure 1 shows a schematic phase diagram, Tc vs. size of the election-phonon interaction, V. Conversely, can we obtain superconductors with a high Tc if we decrease the electron-phonon coupling constant, V, of the charge density wave-type insulators? Based on this idea, we discovered that Nb1−xTaxSe3 became a superconductor when Tc = 4 K, by substituting the Ta at the Nb site to the CDW material, NbSe3 to form Nb1−xTaxSe3 and reducing V1) (Fig. 2).

Phase diagram of the electron-phonon coupling constant V vs. superconducting critical temperature Tc. (N. Tsuda, K. Nasu, J. Fujimori and K. Siratori: Electronic Conduction in Oxide (in Japanese) p. 120.).
Meanwhile, Bednorz and Müller discovered copper oxide superconductors (1986).3) The substance they found was an oxide, called La–Ba–Cu–O. It is said that they first noticed that crystals were distorted because Cu2+ was a Jahn-Teller ion, and they supposed that a high Tc might be obtained by changing the static distortion to a dynamic charge fluctuation. This is very similar to our idea. After that discovery, many groups figured out that this substance was two-dimensional, and that Tc was quickly increased to 160 K just a few years later.
This “revolution within the history of science” eliminated the widely accepted major prejudice that superconductivity is only possible in non magnetic materials. Leaving to other books the dramatic historical details of how a small bud germinated by Bednorz and Müller blossomed into a mainstream concept, here we describe what I did during this period. It is well known that after Bednorz and Müller discovered superconductivity in a copper oxide, a group led by Paul Chu found YBa2Cu3Oy (YBCO).4) Around that time, I started studying the Cu-oxide system in earnest. My idea of how to create a new superconductor centered on the radius of ions. As shown in Table 1, I noticed that all ionic radii of elements in superconductors obtained until then were in the vicinity of 1 Å. Are there any other elements with ionic radii in that vicinity? I found that only four such ions exist: Bi3+, Tl3+, Hg2+, and Cd2+, as listed in Table 1. Surprisingly, these elements (except for Cd), were components of Bi-based, Tl-based, and Hg-based superconductors with a higher Tc.
| Cation | Ionic radius [Å] (6-coordination) |
|---|---|
| Ca2+ | 1.02 |
| Sr2+ | 1.18 |
| Ba2+ | 1.38 |
| Y3+ | 0.91 |
| La3+ | 1.08 |
| Bi3+ | 1.04 |
| Tl3+ | 1.08 |
| Hg2+ | 1.04 |
| Cd2+ | 0.97 |
We now describe here some successful and unsuccessful stories. At that time, we started with the combination of Bi–Sr–Cu–O suggested by Table 1, and immediately obtained a superconductor with Tc = 6 K. Figure 3 shows its crystal structure and electrical resistance.5),6) In a search of superconductors with a higher Tc, we replaced Nd3+, etc. with different valences at the site of Sr2+ for carrier doping. We then found signs of superconductors with Tc = 50 K, and were excitedly working on its single-phase formation. Meanwhile we heard about the discovery of Bi–Sr–Ca–Cu–O with Tc = 77 K and Tc = 105 K by Maeda et al.7) I was disappointed by this discovery, because when one of my students tried to replace Sr2+ with Ca2+, I did not agree with his idea, because Sr2+ and Ca2+ are both divalent elements, and they would only dissolve, leading to nothing interesting chemically. However, it became clear that, in fact, when Sr and Ca are mixed, they enter into completely different sites due to their different ionic radii, resulting in different crystal structures (Fig. 4).

Temperature dependence of resistance in Bi2Sr2CuO6. The inset shows the crystal structure.5)

Layered structure of (a) Bi2Sr2CuO6 and (b) Bi2Sr2CaCu2O8.
Here is another example of our successful and unsuccessful cases. There is a substance, Nd2CuO4−δ, called a fluorite type structure, whose structure was known at that time. Unlike La2CuO4, it does not have oxygen above and below the copper in its crystal structure. We figured out that this substance became metallic when Ce4+ was replaced with Nd3+. Then, one of my students suggested, “let’s mix in Sr2+ in addition to Ce4+”. But I objected to this idea, saying that the number of carriers might be canceled if Sr2+ was mixed with Ce4+. But I remembered my previous mistake, and agreed to try this idea, in fact, a superconductor with a totally new structure emerged.8),9) Figure 5 shows its crystal structure (called “T* structure”) and electrical resistivity.

Shortly after that, however, Tokura et al. discovered a new type of superconductor,10) an electron-doped type, by reducing the oxygen in (Nd1−xCex)2CuO4. This is called the T′ structure. Figure 6 shows the “214”-type crystal structure with a different coordination having a CuO2 plane.

Three types of “214” structure: (a) (La1−xSrx)2CuO4: T structure, (b) (Nd1−xCex)2CuO4: T′ structure, (c) (Nd, Sr, Ce)2CuO4: T* structure.
Meanwhile, some essential characteristics of copper oxide superconductors had become clear.
1. The first is the existence of a CuO2 plane consisting of Cu and O. This “parent compound” is both antiferromagnetic and insulating at the same time. This is called a Mott insulator.
2. The second characteristic is that a superconductor emerges after holes or electrons are introduced into this Mott insulator, and electricity starts flowing. Block layers adhere to the CuO2 plane and adjust the electric charges. Figure 7 is a schematic diagram of these block layers.11) Superconductors with different Tc are generated by replacing these block layers.

Concept of the block layer. CuO2 layers are inserted between block layers.11)
The next logical step is to assemble new superconductors using these block layers. This idea is specifically to create new superconductors by finding new block layers, and combining them with existing block layers. For example, there is a substance with the crystal structure Sr2CuO2(CO3).12) This is a new copper oxide that includes a carbonate group, but this is not a superconductor itself. What should be done to dope this with a carrier? One of my doctor-course students, Masatomo Uehara (currently Yokohama National University), came up with an interesting method. It was to replace CO3−2 with BO3−3, and thus effectively inject a carrier, and generating a new superconductor, Sr2CuO2(CO3)1−x(BO3).13) Figure 8 shows a phase diagram with the boron content x vs. temperature in Sr2CuO2(CO3)1−x(BO3)x. This superconductor is a new copper oxide that includes a carbonate group. Is it possible to create a new type superconductor by combining this crystal structures and those of existing superconductors? It was not as easy as it sounds, but we finally were able to synthesize a new superconductor under high pressure. Figure 9 shows an example of such a superconductor. We were successful in synthesizing 3 new superconductors with Tc = 55 K, 105 K and Tc = 115 K.14),15)

Phase diagram of Sr2CuO4(CO3)1−x(BO3)x. Carriers can be controlled by (BO3) content.13)

Three types of superconductor composed of different block layers including the carbonate layers. (a) Sr2CuO2(CO3)1−x(BO3)x (Tc = 55 K) (b) Sr2CaCu2O4(CO3)1−x(BO3)x (Tc = 105 K) (c) Sr2Ca2CuO4(CO3)1−x (BO3)x (Tc = 115 K).15)
A common characteristic of these superconductors is having a CuO2 plane, which does not essentially introduce any new physics. Is it possible to discover yet more superconductors that belong to new categories? To search for such a situations, we focused on a ladder lattice, which has the structure shown in Fig. 10. The Takano Group of the Institute for Chemical Research at Kyoto University discovered SrCuO2 with a two-leg ladder lattice, and Sr2CuO3 with a three-leg ladder lattice. It was experimentally demonstrated that two-leg ladder lattice substances had spin gaps and three-leg ladder lattice substances had no spin gaps.16) Since then, T.M. Rice,17) E. Dagotto18) and others have pointed out the theoretical possibility of superconductivity in ladder lattice substances. They noted that superconductivity was expressed in the even-numbered ladder lattices and that spin gaps survived.
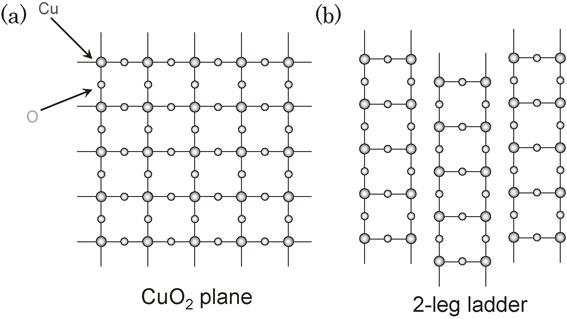
Two types of Cu-O planes. (a) Two-dimensional CuO2 planes, (b) Two-leg ladder Cu2O3 planes.
Are there really such convenient ladder lattice substances? It is amazing how well things work out in nature! Such a substance does exist in reality: Sr14Cu24O41. It is called a ‘telephone-number’ compound due to its peculiar sequence of numbers: 14–24–41. As shown in Fig. 11, this substance’s crystal structure is layered with one-dimensional chain layers on a CuO2 plane and two-leg ladder layers on a Cu2O3 plane. As is evident in its valence of Cu2.25+, many holes already exist in this substance. These holes are mainly localized in one-dimensional chain layers, and their electrical conductivity is similar to that of semiconductors. What should be done to move these holes in the chain layers to ladder layers? The easiest method is to shorten the distance between layers. To achieve this, the Sr site may simply be filled instead with Ca. In fact, when Sr is replaced by Ca in Sr14−xCaxCu24O41, its electrical resistivity decreases. In actual experiments, Ca may replace Sr only up to x = 8.4. Under a high oxygen pressure, however, Ca successfully replaced Sr up to x = 13.6. Unfortunately, however, superconductivity did not emerge. As a last resort, we measured its electrical resistivity under pressure in cooperation with Dr. Mori’s group in the Institute for Solid State Physics (ISSP) at the University of Tokyo, and in fact we found superconductivity of Tc = 12 K at 3 GPa (Fig. 12).19) It was also confirmed by using the single crystal.20)

Crystal structure of telephone number compound Sr14Cu24O41, being composed of 1D-chain layer and 2-leg ladder layer.

(a) Temperature dependence of electrical resistivity in Sr14−xCaxCu24O41. With increasing x (Ca content), resistivity is decreased. (b) Pressure dependence of Sr0.4Ca13.6Cu24O41.84. Superconductivity appears at 3 GPa.19)
During these explorations, we felt that we hit a dead-end with oxide superconductors, and decided to start exploring metal-based ones. Our guiding principle at that time was again the BCS theory. As shown in Eq. [1], Tc is proportional to the Debye temperature (Θ). In general, the lighter are the elements, the higher becomes the Debye temperature. It is expected that superconductivity with lighter elements would have a higher Tc. Thus, we focused on boron (B). I provided Jun Nagamatsu, an undergraduate student at that time, a research topic: to explore compounds of Mg with each of Ti and B. We included Ti because we thought that magnetic ions would play an essential role in some way, and B because it is a light element. Shortly after we began this study, Nagamatsu found a decrease in the magnetization, which was a precursory phenomenon of superconductivity. But we had a hard time to make it a single phase. Finally, Nagamatsu was able to figure out that Ti was not necessary in this superconductivity, and the essential point was to create a substance with a very simple structure, called MgB2.21) Figure 13 shows the electrical resistivity, and magnetic susceptibility, as a function of temperature. Figure 14 shows the crystal structure of MgB2. There were two points that surprised us upon the discovery of this substance. First, MgB2 was a reagent available on the market. It was surprising that a substance commercially available as a reagent is actually a superconductor with a high Tc. This was picked up in the “News and Views” section of Nature with the title “Genie in a bottle” (pp. 23–24).21) The second interesting news was that MgB2’s crystal structure was called the AlB2 structure, which consists of a honeycomb structure of B and a triangular grid of Mg, the layers of which created a characteristic 2-D lattice. In fact, this type of AlB2 structure exists in large numbers (Table 2), and most of these substances were synthesized by B.T. Matthias. Strangely, however, Matthias did not synthesize MgB2. He would have regretted this oversight greatly if he were alive today. MgB2 itself, obtains a high Tc through the electron-phonon interaction coupled with the strong lattice vibration of B, and it is believed that this is a typical (textbook) superconductivity, described by electron-phonon interaction. What is interesting about MgB2 is that the band on the B plane (called σ band) and the band connecting the Boron and Mg ions (called the π band) both contribute to the superconductivity, which is called two-band superconductivity. Figure 15 shows this band structure and the Fermi surface.22)

Electrical resistivity and magnetic susceptibility of MgB2 as a function of the temperature.21)

Crystal structure of MgB2.21)


The band structure and the Fermi surface of MgB2.22)
Thus, MgB2 is a typical BCS-type superconductor, mostly explained by the electron-phonon interaction, but its most interesting point is its applications. The critical temperature (Tc) is not the only parameter to be considered in applications. In general, superconductivity breaks down in a strong enough magnetic field, termed the critical magnetic field (Hc). Superconductivity also breaks if a large amount of electric current is applied, termed the critical current (Jc). In short, superconductivity exists in a region defined by a critical temperature, critical magnetic field and critical current. For the widest range of practical applications, superconductors with high Tc, Hc, Jc are needed. Unlike Tc, Hc and Jc may be increased by various means. For example, Jc and Hc is drastically increased by mixing in small amounts of impurities.
To be used in superconducting magnets and transmission cables, MgB2 must be turned into wires. In that regard, MgB2’s advantages include:
Some of the methods for the creation of MgB2 wires have been proposed based on past knowhow.23) Figure 16 shows two fabrication methods: ① In situ Powder-in-tube (PIT) method and ② Internal Mg diffusion (IMD) method. Recently, the most common method has been Powder-in-Tube (PIT), where a starting powder is stuffed into metal tubes, and processed before and/or after placement in the tube. There are basically two PIT methods. One is an ex-situ method, where pre-processed MgB2 powder is stuffed into metal tubes, such as stainless steel. The other is an in-situ method, where a mixed powder of Mg and B is stuffed in a tube to fabricate tapes, which are then heat-treated. In the ex-situ method, the greatest advantage is that a worthwhile Jc may be obtained, even without the heat-treatment process. A further heat treatment (annealing) improves the binding between crystal grains, leading to a higher Jc. In the “in-situ” method, the reactivates of Mg and B are enhanced with a heat treatment, and at the same time, unlike in the ex-situ method, there is our advantage that the crystal grains are rarely oriented.

Two fabrication methods to make a MgB2 wire. 1) In situ powder-in-tube (PIT) method. 2) Internal Mg diffusion (IMD) method.23)
Another effective method to increase the MgB2 core density is an internal Mg diffusion (IMD) process.24) The IMD method is believed to provide a higher Jc due to our improved connectivity of MgB2. The starting materials used are also important. Recently, there have been many reports that the characteristics of Jc are improved when a trace amount of minute impurities is added to a mixed powder of Mg and B. Currently, the most effective method is to add minute SiC particles that are in the nanometer range. Figure 17 shows the temperature dependence of Bc2 in MgB2 tapes with no additives, and those with SiC added. In wires with SiC added, Bc2 for 4.2 K was as high as 30 T. This value is equal to or greater than the value of Bc2 in Nb3Sn wires. The Bc2 in 20 K wires ranges up to 11 T. This shows that currently used utility wires may be replaced with MgB2.

Temperature dependence of Bc2 in MgB2 wires.23)
Naturally, for use in magnet wires, the magnetic field dependence of Jc is an important factor. Figure 18 shows the present status of MgB2 wires prepared by both the PIT and IMD methods. As shown in Fig. 18, for the practical level at 20 K and 2 T, Jc in PIT wire keeps Jc ∼ 105 A/cm2. Unfortunately, Jc reduces to 1.2 × 104 A/cm2 at 20 K and 5 T. However, there has recently been rapid progress in this area. By using the IMD method, it reached to almost 9 × 104 A/cm2 at 20 K and 5 T. The future is promising.

Present status of critical current density Jc (A/cm2) vs. magnetic field B (T).23)
Since discovering the MgB2 superconductor, we have conducted broad explorations of superconductors that include light elements, such as boron (B) and carbon (C). As a result, we found that Y2C3 is a superconductor (Tc = 18 K) synthesized under high pressure. Figure 19 shows the temperature dependence of the resistivity and the susceptibility at ambient pressure. Tc is controllable by a synthesis condition.25)–27) Afterwards, we found that this substance had already been reported by Krupka et al.28) as having a maximum Tc of 11.5 K. Figure 20 shows its crystal structure to be the Pu2C3-type, with a body-centered cubic structure (b.c.c.). Its Y atoms line up along the ⟨111⟩ direction, and its carbon atoms form dimers, with an extremely short C-C bond length. It is expected that its C-C stretching phonon mode is very high. Therefore, the relatively high Tc of Y2C3 is thought to be the result of electron-phonon coupling between high-frequency phonons and the C-C antibonding state in the vicinity of the Fermi level. A characteristic of both Y2C3 and La2C3, which has the same structure as Y2C3, is that they are superconductors with two gaps. This was first suggested by NMR measurement of spin-lattice relaxation.29) It was then clarified further in a µSR measurement.30) Figure 21 shows the temperature dependence of the muon spin relaxation rates of La2C3 and Y2C3. Solid and dashed lines are fitted with the s-wave’s phenomenological double-gap model.30) It was theoretically suggested that in the crystal structure of Y2C3, a strong spin-orbit interaction might lead to a mixed state of symmetric isotropic and anisotropic superconducting properties due to the lack of a space inversion symmetry. It was reported later that Bc2 is very high, at approximately 30 Tesla, which may be related to the above state.

Crystal structure of Y2C3 to be the Pu2C3-type with (b.c.c.) structure.

Temperature dependence of the muon spin relaxation rates of La2C3 and Y2C3. Solid and dashed lines are fitted with the s-wave’s phenomenologial double-gap model.30)
Diamonds, famously known as jewels, do not allow the flow of electricity, as is evident from their sparking. Surprisingly, however, it was discovered that diamonds become superconductive if carrier-doped (Tc = 3 K).31) By making films even thinner, its Tc was raised to 12 K.32) The superconducting transition Tc = 0.35 K was also reported when B was doped into silicon (Si), with a similar crystal structure.33) Both substances are typical wide-gap semiconductors with the well-known diamond structure. If highly concentrated hole carriers are doped to those wide-gap semiconductors, superconductivity will be created. Since then, many theoretical studies have been actively conducted, indicating the possibility of the emergence of superconductivity at considerably higher temperatures, depending on the doped B atoms’ order or alignment.34)
We tried to create superconductivity by B replacement (hole doping) in SiC, with characteristics similar to those of diamonds and Si, and discovered that its bulk was a superconductor with Tc = 1.4 K.35) We also tried to use aluminum (Al) as a hole dopant in the same manner, and verified a superconducting transition at a similar Tc (≒ 1.4 K).36) In the following sections, the basic physical properties that have been discovered so far are explained.
(2) Crystal structure of SiC.SiC has a crystal structure in which C and Si are basically placed at even intervals in a diamond-type structure. However, when one looks precisely, many different structures emerge (polytype: crystal polymorphism), depending on the type of lamination layer formed. Although 200 or more polytypes have been verified currently, 3C-, 4H-, 6H-, and 15R-SiC (Ramsdell notation system) are the most important in applications, and have a high probability. In this notation system, the first number refers to the number of Si-C unit layers included in the layer direction (c, axis direction) during one cycle; C, H and R are the initial letters of crystal systems (C: cubic, H: hexagonal, R: rhombohedral). The zincblende structure that often emerges in other semiconductors is expressed as 3C, and the wurtzite structure is 2H. All band structures are of the indirect transition type, the same as that of Si. Among these polytypes, we focused on 3C-SiC and 6H-SiC (Fig. 22), and attempted hole doping in each of them.

Crystal structure of the 3C-SiC and 6H-SiC, respectively.
Figure 23 shows the temperature dependence of the electrical resistivity in SiC samples (3C-SiC:B, 6H-SiC:B), where B was doped into 3C-SiC and 6H-SiC. In both, though the Tc values are low (approximately 1.4 K), a sharp superconducting transition can be observed. Also, in these transitions, substantial Meissner diamagnetism has been observed in measurements of the DC magnetic susceptibility (Fig. 23), and specific-heat jumps have been observed, confirming bulk superconductivity. It is considered that differences in Tc and the residual resistance between these substances show their disparity in reactivity, since 3C-SiC is in its low-temperature stabilized phase and 6H-SiC is in its high-temperature stabilized phase.

Temperature dependences of the electrical resistivities and magnetic susceptibilities.36)
Figure 24 shows a magnetic field vs. temperature phase diagram of the superconducting state, as determined by measuring the electrical resistivity with changing temperature under a constant magnetic field (T-scan), and also with a changing magnetic field under a constant temperature (H-scan). As shown in Fig. 24, Tc during the cooling process starting from T > Tc and the Tc observed during the warming process do not match. This strongly suggests that B-doped SiC is a Type I superconductor. In general, simple elements, such as tin (Sn) and lead (Pb), become Type I superconductor, and Type I superconductivity in a chemical compound is very rare. The above-mentioned B-doped diamonds and B-doped Si are reported to be Type II superconductors.

H-T phase diagram for (a) B-doped 3C-SiC(3C-SiC:B) and (b) B-doped 6H-SiC(6H-SiC:B), determined from the onset of superconductivity during a T-scan and H-scan of resistivity.
Figure 25 shows the temperature vs. electrical resistivity and magnetic susceptibility in SiC samples (3C-SiC:Al), where 3C-SiC was doped with Al. Tc was approximately 1.5 K, almost equal to that of the above B-doped SiC, for which superconductivity can be verified. Also, in this transition, substantial Meissner diamagnetism has also been observed in measurements of the DC magnetic susceptibility, and a specific-heat jump has also been observed, confirming bulk superconductivity.

Temperature dependences of the electrical resistivity and magnetic susceptibility in SiC(3C-SiC:Al), where 3C-SiC was doped with Al.36)
Figure 26 shows a magnetic field and temperature phase diagram of the superconducting state, as determined by measuring the electrical resistivity with changing the temperature under a constant magnetic field, and also with changing magnetic field under a constant temperature. Unlike the above B-doped SiC, under a finite magnetic field, that observed during the cooling process matched the Tc observed during the warming process, suggesting that this is a Type II superconductor, the same as B-doped diamond and B-doped Si.

Temperature dependence of the upper critical field Hc2 and the irreversibility field Hirr in 3C-SiC:Al.36)
The superconductivity of B-doped SiC involves almost the same carrier density as the superconductivity in B-doped diamond and B-doped Si, and its crystal structure is almost the same as that of diamond. It is very interesting to understand why only B-doped SiC is a Type I superconductor. Our experimental results may contribute to a qualitative understanding of this question by using the impurity level formed in semiconductors.
Table 3 summarizes the normal-state properties, including the impurity levels (EA), and also the superconducting parameters of B-doped diamond (C:B), B-doped Si (Si:B), B-doped SiC (SiC:B), and Al-doped SiC (SiC:Al).
| SiC:Al | SiC:B | C:B | Si:B | |
|---|---|---|---|---|
| n (cm−3) | 7.06 × 1020 | 1.91 × 1021 | 1.80 × 1021 | 2.80 × 1021 |
| γn (mJ/molK2) | 0.35 | 0.294 | 0.113 | — |
| ρ0 (mΩcm) | 0.746 | 0.06 | 2.5 | 0.13 |
| RRR | 5.3 | 10.0 | 0.9 | 1.2 |
| Tc(onset) (K) | 1.5 | 1.45 | 4.50 | 0.35 |
| Hc(0) (Oe) | — | 115 | — | — |
| Hsc(0) (Oe) | — | 80 | — | — |
| Hc2(0) (Oe) | 432 | — | 4.2 × 104 | 4000 |
| kF (nm−1) | 2.8 | 3.8 | 3.8 | — |
| m* (mel) | 2.0 | 1.2 | 1.7 | — |
| νF (m/s) | 1.6 × 105 | 3.8 × 105 | — | — |
| l (nm) | 2.2 | 14 | 0.34 | — |
| ξ(0) (nm) | 87 | 360 | 80(9) | (20) |
| λ(0) (nm) | 281 | 130 | 160 | — |
| κGL | 3.2 | 0.35 | 2(18) | — |
| EA (eV) | ∼0.25 | ∼0.29 | 0.37 | 0.045 |
Although B-doped Si has been reported as forming Type II superconductors, recent studies suggest that they may be Type I superconductors, as revealed by samples with improved quality. B-doped Si’s Type I superconductivity may have been hidden due to the crystals’ irregularities and/or disorder, resulting in their being reported as Type II superconductivity despite the fact that it is essentially Type I superconductivity. What is observed from these parameters is that the disorder of the impurity or its level has a large impact if the acceptor level is deep, and leads to Type II superconductivity. SiC:Al may be positioned on the border of Type I and Type II superconductivities in hole-doped superconductors. Also, since the state density in the vicinity of SiC’s Fermi level is mainly due to the Si structure, it is inferred that the Si may be more important than the C for superconductivity. Based on structural analysis results, it is considered that Al may show Type II superconductivity due to the stronger impact of any disorder of Al, since it is supposed that B is then transposed to the C site and Al is transposed to the Si site.
4-4. New superconductor with a cage-type structure — R5T6Sn18 (R = Sc, Y, Lu; T = Rh, Ir).In this section, a new superconductor with a cage-type structure that we have worked on is briefly explained. R5T6Sn18 (R = Sc, Y, Lu; T = Rh, Ir) was first discovered by Remeika et al.37) We, however, discovered a totally new experimental fact by systematically synthesizing single-crystal samples of it.38) R5T6Sn18 has a cage-type structure consisting of R5T6Sn18 (Fig. 27). This is a group in which the superconducting state changes as the R element included in the cage changes. The superconducting transition temperatures are Tc = 5, 3, or 4 K, for R = Sc, Y, or Lu, respectively. Sc, with the smallest ionic radius, has the highest Tc (Fig. 28). The most noteworthy characteristic of this group is that the superconducting gaps change substantially depending on the rare-earth ion, R.38) The magnetic field dependencies of the quasiparticle state densities (γ) resulting from specific-heat measurements in Lu5Ir6Sn18 and Lu5Rh6Sn18 at various temperatures are γ ∼ H0.90 and γ ∼ H1.1, respectively, representing isotropic superconducting gaps.38) While these isotropic superconducting gaps may be explained by the BCS theory,39) the magnetic-field dependencies of γ in Y5Ir6Sn18 and Y5Rh6Sn18 are γ − H0.58 and γ − H0.56, respectively, representing anisotropic superconducting gaps38),39) (Fig. 29).

Cage-type crystal structure of R5T6Sn18 (R = Sc, Y, Lu; T = Rh, Ir).38)

Temperature dependence of the magnetic susceptibility in R5Rh6Sn18 (R: Sc, Y and Lu).38)

Quasiparticle state density (γ) vs. magnetic field.38)
Table 4 summarizes the normal and superconducting parameters. Unfortunately, the physical reason why such a major physical property changes so substantially when rare-earth elements are changed is not yet clear.
| Space group | Sc5Rh6Sn18 | Sc5Ir6Sn18 | Y5Rh6Sn18 | Y5Ir6Sn18 | Lu5Rh6Sn18 | Lu5Ir6Sn18 |
|---|---|---|---|---|---|---|
| I41/acd | I41/acd | I41/acd | Fm3m | I41/acd | I41/acd | |
| Gap asymmetry | isotropic | isotropic | anisotropic | anisotropic | isotropic | isotropic |
| a (nm) | 1.3601 | 1.3595 | 1.3792 | 1.3735 | 1.3671 | 1.3602 |
| c (nm) | 2.7198 | 2.7180 | 2.7498 | — | 2.7330 | 2.7315 |
| Tc (K) | 5.0 | 1.0 | 3.0 | 2.1 | 4.0 | 3.0 |
| μ0Hc(0) (mT) | 99.1(1) | 12.2(1) | 50.1(4) | 30.4(1) | 75.0(2) | 47.6(1) |
| μ0Hc1(0) (mT) | 5.64(1) | — | 2.49(2) | 1.46(1) | 4.38(1) | 1.87(2) |
| μ0Hc2(0) (T) | 7.24(5) | — | 4.21(6) | 2.91(6) | 5.58(5) | 3.90(10) |
| λ(0) (nm) | 34.2(1) | — | 51.4(2) | 67.2(1) | 38.8(1) | 59.2(2) |
| ξ(0) (nm) | 6.74(1) | — | 8.84(4) | 15.9(1) | 7.68(2) | 13.0(1) |
| κ(0) | 51.7(1) | — | 59.1(4) | 62.1(1) | 50.5(1) | 66.8(1) |
| γ (mJ/(mol·K2)) | 51.0(4) | 27.3(4) | 37.8(4) | 32.1(2) | 49.1(4) | 31.9(1) |
| ΘD (K) | 276(2) | 246(2) | 185(1) | 212(2) | 158(1) | 193 |
| Δ(0) (meV) | 0.990(3) | 0.140(1) | — | — | 0.719(2) | 0.544(3) |
| ΔCe/γnTc | 2.55(36) | 1.39 | 1.96 | 1.68 | 2.02 | 1.83 |
| 2Δ(0)/kBTc | 4.26(3) | 3.26(6) | — | — | 4.12(2) | 4.07(5) |
In the previous sections, the main topics concerning the new superconductors discovered in our laboratory are described, which can be categorized into two groups:
Finally, we summarize here all of the superconductors discovered in our laboratory (Table 5).
| Cu-oxide based new superconductors | |||
|---|---|---|---|
| Year | Superconductor | Tc (K) | Reference |
| 1987 | Bi-Sr-Cu-O | 6 | 5), 6) |
| 1988 | Nd-Ce-Sr-Cu-O | 28 | 8), 9) |
| 1989 | (Eu,Ce)-(Ba,Ln)-Cu-O (Ln = Nd, Sm, Eu) | 43 | 40) |
| 1992 | (Y,Ca)-Sr-Cu(CO3)-O | 63 | 41) |
| 1992 | (Bi,Pb)-Sr-Cu-(CO3)-O | 41, 54 | 42) |
| 1993 | Sr-Ca-Cu-(CO3)-(BO3)-O | 33, 55, 105, 115 | 14), 15) |
| 1993 | Tl-(Ba,Sr)-Cu-(CO3)-O | 70 | 43) |
| 1993 | Hg-Ba-Sr-Cu-(CO3)-O | 66 | 44) |
| 1994 | Ba-Ca-Cu-(CO3)-(BO3)-O | 120 | 45) |
| 1994 | (Ca,Na)-Ca-Cu-O-Cl | 49 | 46) |
| 1995 | (Ca,A)-Cu-O-Br (A = Na, K) | 19 | 47) |
| 1996 | Ba-Ca-Cu-O-F | 38, 106, 108 | |
| 1996 | Sr-Ca-Cu-O(14-24-41) | 12 | 19) |
| 1998 | Cu-Sr-(Y,Ce)-Cu-O | 43 | 48) |
| 1999 | Ru-Sr-Y-Cu-O | 40 | 49) |
| Metal-based new superconductors | |||
|---|---|---|---|
| Year | Superconductor | Tc (K) | Reference |
| 1984 | (Nb,Ta)Se3 | 4 | 1) |
| 2001 | MgB2 | 39 | 21) |
| 2003 | Re-B | 5 | 50) |
| 2004 | Y2C3 | 18 | 25) |
| 2006 | (W,Mo)-Re-(B,C) | 7, 8 | 51) |
| 2007 | NaAlSi | 7 | 62) |
| 2007 | SiC:B | 1.4 | 35) |
| 2008 | SiC:Al | 1.5 | 36) |
| 2008 | AlNx | 2.8 | |
| 2009 | W5Si3 | 2.8 | 52) |
| 2010 | YSn3 | 7 | 53) |
| 2010 | Y3Pt4Ge6 | 2.6 | 54) |
| 2011 | W5SiB2 | 5.8 | 55) |
| 2012 | (Ta,W)5SiB2 | 6.5 | 56) |
| 2012 | KAlX (X = Si, Ge) | 3.5, 4 | |
| 2012 | AE(TM,Si)2 (AE = Ca, Sr, Ba; TM = Ni, Pd, Pt, Cu, Ag, Au) | 0.9–3.5 | 57) |
| 2013 | KSn2 | 3.2 | 58) |
| 2013 | A8(Al,Ge)46 | 4, 4.4 | 59) |
| 2013 | Lu2SnC | 5 | 60) |
| 2014 | Cr2Re3B | 6 | 61) |
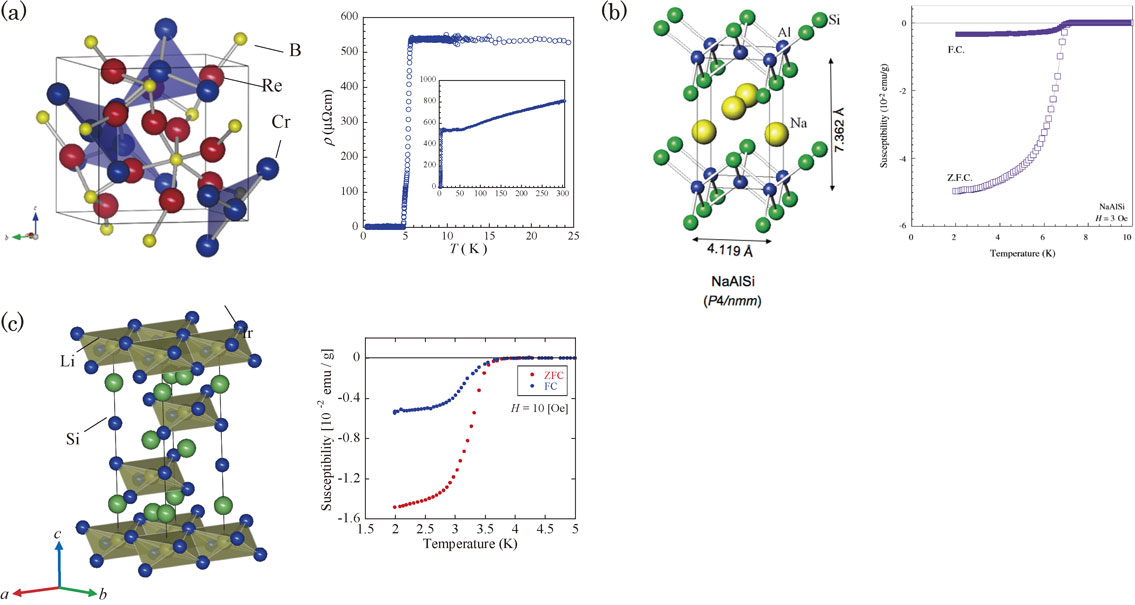
From the Table 5, we chose typical examples of superconductivities as well as crystal structures in Cr2Re3B,61) NaAlSi62) and LixIrS2 (Fig. 30).
In a previous section, we described what we have done. Next is our future prospects concerning what we should attack. We present here several candidates to achieve higher-Tc (Tc ≥ 100 K) superconductors.
(1) Hydride system.The first one is the hydride system. Hydride system has long been expected to be a candidate of room-temperature superconductor. Unfortunately, however, only a moderate Tc ∼ 17 K was observed in the Pd hydrate experimentally. Recently, much attention has been paid to superconductivity at 203 K at ultra-high pressure in SH3.63) Subsequently, superconductivity above 260 K (near room temperature!) has been discovered in LaH10.64),65) Disappointedly, it has only been achieved under ultra-high pressure at around 200 GPa, which is not a practical pressure. The next step is to discover a new hydride system, and to realize a high-Tc superconductor under ambient pressure.
(2) Superconductivity in 4d, 5d system.The second big problem is that “Is there any other existing transition metal (except for Cu, Fe) oxide/chalcogenide superconducto? In particular, a high-Tc superconductor of the 4d, 5d system is unexplored?”. This will be discussed in the next section.
(3) High-Tc superconductor originated from charge fluctuation or valence skipping system.It is well known that the origin of a high-Tc superconductor in Cu-oxide is due to magnetic fluctuation instead of some election-phonon interaction. In this sense, the charge fluctuation is another candidate to produce high-Tc superconductivity, for example Ba1−xKxBiO3 (Tc ∼ 30 K) is believed to be a charge fluctuation-mediated superconductor.
However, why can we not get a higher-Tc (Tc ≥ 100 K) superconductor mediated by charge fluctuation? This is a third big problem.
5-3. Our present work.In this section, we briefly describe what we are presently working on.
Recently, much attention has been paid to superconductivity in the Iridate system. If superconductivity is realized in this system with the strong spin-orbit coupling (S.O.C), it will open the new dimension in condensed-matter physics. In particular, Sr2IrO4/Ba2IrO4 has been predicted to be a high-temperature superconductor upon electron doping,66) since it highly resembles the cuprate in the crystal structure and magnetic coupling constant. Particularly, the remarkable resemblance between Sr2IrO4/Ba2IrO4 and La2CuO4 makes a good candidate to expect unconventional HTSC in Sr2IrO4/Ba2IrO4 (Fig. 31). Indeed:

(a) Crystal structure of La2CuO4 and Sr2IrO4. (b) Schematic diagram of the 5d energy levels split by the crystal field (Δ), spin–orbit coupling (SOC), and on-site Coulomb repulsion (U) leading to the formation of unoccupied (upper) and occupied (lower) Hubbard bands at around the Fermi level from the Jeff = 1/2 band and the fully occupied Jeff = 3/2 band.
In particular, Terashima et al.69) demonstrated that the d-wave gapped state approaches the Fermi energy as the doped carrier increases, which shows a striking similarity with those observed for underdoped cuprate, suggesting that superconductivity can be realized with increasing the carrier concentration (Fig. 32).

(a)–(c) Second derivative of ARPES intensity plots as a function of the binding energy and wave vector on Sr2−xLaxIrO4 (x = 0, 0.04 and 0.08) measured along the k direction. (d) Schematic graph of the doping-induced change in the electronic structure of Sr2−xLaxIrO4 derived from our ARPES study.69)
Our conclusions are summarized as follows at the present stage:
In conclusion, however, we can not get a bulk superconductivity, probably due to insufficient carriers in the IrO2 planes.

Magnetic phase diagram of Sr2−xLaxIrO4 determined from magnetic susceptibility and µSR measurements.70)
I deeply acknowledge my colleagues, Drs. H. Sawa, M. Uehara, T. Muranaka, Y. Zenitani, S. Akutagawa, K. Kawashima, K. Horigane, Z.A. Ren, S. Kuroiwa, N. Kase and all our members in my laboratory for our collaborations. In particular, I deeply acknowledge Dr. Kumakura for the discussion of applications of MgB2. Finally, we thank to K. Horigane, M. Akimitsu, E. Sakamoto and H. Tanaka for assisting me to prepare the manuscript.

Jun Akimitsu was born in Hiroshima Prefecture in 1939. He graduated from the University of Tokyo and also received a Ph.D. from there. He became Research Associate at Institute for the Solid State Physics (ISSP), the University of Tokyo (1970–1976). He moved to Aoyama Gakuin University as Associate Professor (1976–1982), and later became Professor (1982–2015). He became the Dean of the Center for Advanced Technology, Aoyama Gakuin University (1998–2015). Meanwhile, he was a visiting professor at MIT (U.S.), and Monash University (Australia) and Guest Scientist at Brookhaven National Laboratory. After retirement from Aoyama Gakuin University, he moved to Research Institute for Interdisciplinary Science, Okayama University, in 2015. He is presently Professor (Special Appointment) of Okayama University.
He has received the following Awards; 1) 1997, Superconducting Science and Technology Award (first: For the discovery of Superconductivity in a ladder compound); 2) 1998, Nishina Memorial Award; 3) 2001, Medal with Purple Ribbon; 4) 2002, The Asahi Prize; 5) 2002, Masumoto Hakaru Award; 6) 2002, Superconducting Science and Technology Award (second: For the discovery of MgB2); 7) 2002, Magnetic Society of Japan Award; 8) 2003, Bernd T. Matthias Prize; 9) 2007, Nishikawa Award; 10) 2008, James C. McGroddy Prize for New Materials; 11) 2012, Special Prize from the Japanese Society for Neutron Society; 12) 2014, The order of the Sacred Treasure; 13) 2019, Superconductivity Science and Technology Award (third: For distinguished contribution to the Discovery of New Superconductors).