2020 Volume 96 Issue 5 Pages 171-179
2020 Volume 96 Issue 5 Pages 171-179
It is generally thought that younger people are more susceptible to cancer development after exposure to ionizing radiation in reference to epidemiological studies and animal experiments. However, little is known about the age-dependent alteration in DNA repair ability. In the present study, we examined the expression levels of proteins involved in the repair of DNA double-strand breaks through non-homologous end joining (NHEJ), i.e., DNA-dependent protein kinase catalytic subunit (DNA-PKcs), X-ray repair cross-complementing 4 (XRCC4) and XRCC4-like factor (XLF). We found that the expression of DNA-PKcs in brain tissues was higher in neonatal mice (1 week after birth) than in young adult mice (7 weeks after birth). In association with this, DNA double-strand breaks were repaired more rapidly in the brain tissues of neonatal mice than in those of young adult mice. The current results suggested a possible role for DNA-PKcs protecting developing brain tissues from DNA double-strand breaks.
It is generally thought that younger people are more susceptible to cancer development after exposure to ionizing radiation in reference to epidemiological studies of atomic bomb survivors of Hiroshima and Nagasaki.1)–4) There are also a number of studies showing age-dependent changes in radiation-induced carcinogenesis in animal experiments. Sasaki and colleagues studied carcinogenesis as well as life-shortening in mice irradiated at various ages.5),6) It was found that the excess relative risk for solid tumors was higher when mice were irradiated at 0 or 7 days after birth than when they were irradiated at 35, 105, or 365 days after birth or in utero (day 17). There was also a significant change in the spectrum of cancers depending on the age at the time of irradiation: a higher incidence of liver and lung tumors were observed when mice were irradiated at 0 or 7 days after birth, whereas the incidences of malignant lymphoma of lymphocytic type and histiocytic type were higher when mice were irradiated at 35 days after birth or later. Pazzaglia and colleagues showed that medulloblastoma in the Patched 1 (Ptch1) heterozygous background was induced at a high incidence within a narrow window of time centered on the days around birth.7)–9) Kokubo et al. reported that renal cell carcinoma in Tuberous Sclerosis Complex 2 (Tsc2) heterozygous rats was most evident when the rats were irradiated at perinatal ages, being maximal at gestation day 19.10)
DNA double-strand breaks (DSBs) are thought the most deleterious type of DNA damage among those induced by radiation and most intimately associated with the biological effects of radiation, including carcinogenesis. In eukaryotic cells, DSBs are repaired mainly through two pathways, i.e. non-homologous end joining (NHEJ) and homologous recombination (HR).11) NHEJ is generally considered less accurate than HR. Nevertheless, NHEJ has a merit of operating throughout the cell cycle, whereas HR is available only in late S and G2 phases, where sister chromatids exist. NHEJ also plays an important role in V(D)J recombination to generate the diversity of immunoglobulins and T cell receptors in immune systems. In NHEJ, Ku heterodimer, which is comprised of Ku70 and Ku86 (also known as Ku80), first binds to the DNA ends and, in turn, recruits DNA-PKcs. Two DNA ends are finally joined by DNA ligase IV (LIG4). Other essential components of NHEJ are XRCC4, XLF, and PAXX, which share similarity in structure and are thought to comprise a molecular superfamily. XRCC4 is required for the stabilization and nuclear localization of LIG4. XLF is thought to support LIG4 activity toward incompatible or mismatched DNA ends. PAXX is shown to interact with Ku and stabilizes NHEJ machinery.
The abundance of DNA repair proteins may be an important determinant of genomic stability, which in turn prevents carcinogenesis. There have been only a few studies on age-dependent alteration in DNA repair ability and the abundance of DNA repair proteins in mice. Hudson et al.12) studied the induction and repair of DSBs after X-ray irradiation in various tissues of mice at different ages. They showed that younger mice were more susceptible to DSBs by γ-irradiation and had more persistent DSBs than older mice, especially in the spleen and thymus. They also examined the expression levels of Ku70, Rad51, which is involved in HR, and DNA polymerase β, which is involved in base excision repair and noted some change in the abundance of these proteins after irradiation. In their later study,13) they focused on the spleen and examined DSBs and the expression levels of proteins involved in various DNA repair pathways, i.e., DNA polymerase δ, DNA polymerase ε, APE1, and MSH2, in addition to Rad51, Ku70, and DNA polymerase β at different ages (2 weeks, 2 months, and 18 months after birth).
In this study, we examined the age-dependency of the expression level of proteins that are involved in DSB repair through NHEJ, i.e., DNA-PKcs, XRCC4, and XLF. We focused on brain tissues, because NHEJ factors are implicated in the development of the brain (see discussion). It was found that DNA-PKcs was more abundant in the cerebellum and cerebrum of neonatal mice (1 week after birth) than in those of young adult mice (7 weeks after birth) in correlation with DSB repair ability.
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of the National Institute of Radiological Sciences (NIRS), National Institutes for Quantum and Radiological Science and Technology (QST) and conducted in accordance with national and institutional guidelines. Male and female offsprings of crosses between C57BL/6NCrlCrlj female mice and C3H/HeNCrlCrlj male mice (B6C3F1) (Charles River Japan) were used for experiments. We chose this genetic background because it has been used in a number of studies on age-dependency of radiation-induced carcinogenesis by Sasaki and colleagues5),6) and us.14),15) Note that variations in the Prkdc gene encoding DNA-PKcs, c.T6,418C and c.G11,530A, resulting p.C2,140R and p.V3,844M, respectively, have been reported.16),17) Both the C57BL and C3H strains have alleles with T6,418 and G11,530, which are linked to higher DNA-PKcs stability and DNA-PK activity.16),17) All mice were maintained in specific-pathogen-free conditions on a 12:12 hr light-dark schedule, at 23 ± 2 °C with 50 ± 10% humidity, on a standard laboratory diet with water ad libitum.14),15) At 1 or 7 weeks of age, mice were euthanized and autopsied. Where indicated, mice were exposed to 4 Gy of whole-body γ-ray irradiation using a 137Cs source Gammacell (Nordion International, Ottawa, Canada) at a dose rate of 0.57 Gy/min. For immunohistochemistry, paraffin-embedded tissue specimens, which had been archived in J-SHARE (Japan Storehouse of Animal Radiobiology Experiments) at NIRS, QST,18) were used.
Western blotting.Organ of mice were mashed in RIPA buffer (Nacalai Tesque, Kyoto, Japan) at the ratio of 3 ml buffer per 1 g organ weight using a disposable homogenizer BioMasher II (Nippi, Tokyo, Japan). After keeping at 4 °C for 30 min, the mash was centrifuged at 20,000 g for 10 minutes and the clear supernatant was isolated. The supernatant was mixed with three volumes of water, and four volumes of 2 × SDS-PAGE loading buffer (125 mM tris(hydroxymethyl)aminomethane, adjusted to pH 6.8 with HCl, 4% w/v sodium lauryl sulfate, 20% v/v glycerol, 5% v/v 2-mercaptoethanol, 0.02% w/v bromophenol blue, 0.01% w/v crystal violet) and heated at 100 °C for 10 minutes.
Western blotting procedures essentially followed our earlier publication.19) Separating gels containing 7.5% polyacrylamide were used for the analysis of DNA-PKcs, whereas separating gels containing 10% polyacrylamide were used for the analysis of other proteins. The primary antibodies used were anti-DNA-PKcs mouse monoclonal antibody clone Ab-4 (ThermoFisher, used at 1:1,000 dilution), anti-XLF rabbit polyclonal antibody X4629 (Sigma-Aldrich, used at 1:1,000 dilution), anti-XRCC4 rabbit polyclonal antibody (generated in our earlier study;20) used at 1:500 dilution) and anti-β-tubulin rabbit polyclonal antibody 39645 (GeneTex, used at 1:1,000 dilution). The secondary antibodies used were anti-mouse immunoglobulin goat polyclonal antibody conjugated with horseradish peroxidase P0447 (DAKO, used at 1:3,000 dilution) for DNA-PKcs and anti-rabbit immunoglobulins swine polyclonal antibody conjugated with horseradish peroxidase P099 (DAKO, used at 1:1,500 dilution) for XLF, XRCC4, and PCNA. Western Blotting Substrate Plus (Pierce) and Hyperfilm MP (GE Healthcare) were used for the visualization of the immunocomplexes. For quantification, the blots were examined using ImageQuant 350 (GE Healthcare). Statistical significance was evaluated using one-tailed t-tests, assuming unequal distributions in the two groups being compared.
Immunohistochemistry.Resected brain tissue of mice was rinsed in ice-cold phosphate-buffered saline, fixed in 10% neutral buffered formalin for approximately 12 hr, and then embedded in paraffin. The paraffin-embedded tissues were cut to a thickness of 3 µm on a glass slide. Immunohistochemical procedure essentially followed our earlier publication.15) The primary antibodies used were anti-DNA-PKcs mouse monoclonal antibody clone Ab-4 (ThermoFisher, used at 1:1,000 dilution), anti-DNA-PKcs phospho-Ser2056 rabbit polyclonal antibody ab18192 (Abcam, used at 1:1,000 dilution), anti-γ-H2AX (H2AX phospho-Ser139) mouse monoclonal antibody 2F3 (BioLegend, used at 1:1,000 dilution) and anti-cleaved-caspase-3 (Asp175) rabbit polyclonal antibody #9661 (Cell Signaling Technology, used at 1:800 dilution).
We first compared the expression level of NHEJ-related proteins in the brain tissues of mice at 1 week of age (1 w) and 7 weeks of age (7 w) using western blotting. For each age, 2 male mice and 2 female mice were examined. It was found that the expression level of DNA-PKcs was much higher in the cerebrum (2.5-fold, p = 0.031) and the cerebellum (6.0-fold, p = 0.012) of 1 w mice than in those of 7 w mice (Fig. 1). In contrast, the expression level of XLF was higher in the cerebrum (8.7-fold, p = 0.009) and the cerebellum (14.2-fold, p = 0.017) of 7 w mice than in those of 1 w mice. The expression level of XRCC4 was not statistically different between 1 w and 7 w mice in the same brain regions. The expression level of β-tubulin appeared somewhat higher in the cerebrum (1.3-fold, p = 0.142) and the cerebellum (1.4-fold, p = 0.042) of 1 w mice than in those of 7 w mice, although the difference was much smaller than in the case of DNA-PKcs. We did not find consistent and substantial differences in the expression of these proteins between male mice and female mice at the same age.
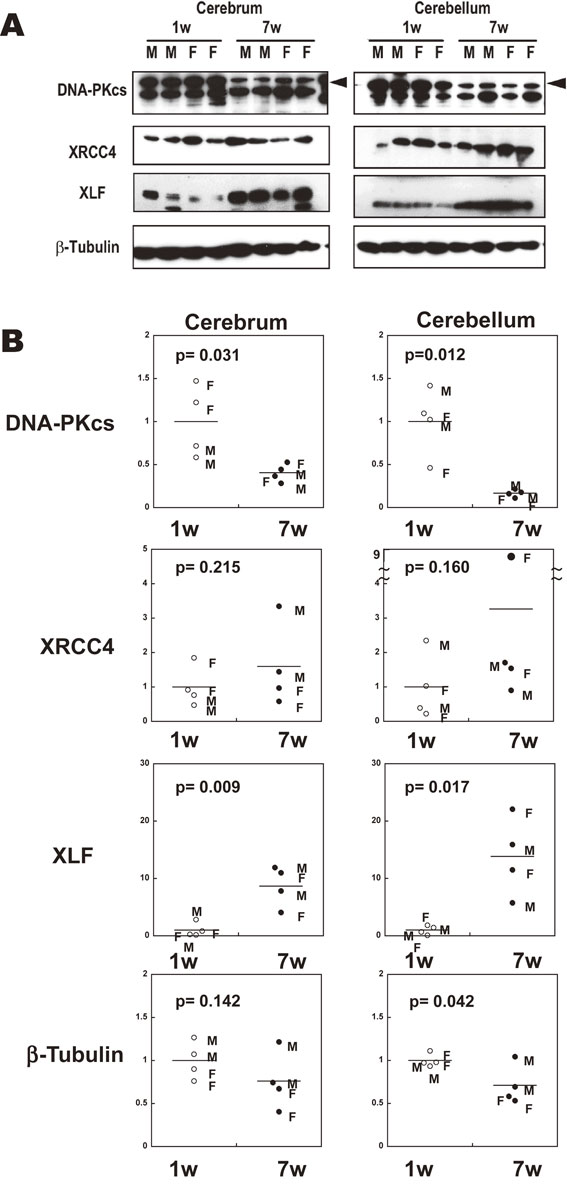
A, western blotting analyses of NHEJ-related proteins in the cerebrum and cerebellum of mice at different ages. Organs were collected from 4 mice (2 male and 2 female) at 1 week of age (1 w) and 7 weeks of age (7 w) and examined by western blotting using antibodies against DNA-PKcs, XRCC4, and XLF, as indicated. Arrowheads in the top panels point to DNA-PKcs. β-Tubulin was examined as the loading control. B, quantitative analysis. The integrated density of each of the band was normalized as the average of 4 animals in 1 w group. Labels “F” and “M” indicate female and male animals, respectively. Horizontal bars show the average of each group. Statistical significance (p value) was evaluated using a one-tailed t-test, assuming unequal distributions of the two groups being compared.
To examine the spatial expression patterns of DNA-PKcs, sections of brain tissue were prepared from 1 w and 7 w mice with or without γ-ray irradiation and examined by immunostaining using anti-DNA-PKcs antibody. In agreement with the results of the above western blotting experiments, DNA-PKcs expression in the cerebrum and cerebellum was higher in 1 w mice than in 7 w mice (Fig. 2). To assess the functionality of DNA-PKcs, the sections were also examined for the autophosphorylation of DNA-PKcs at Ser2056 (pS2056).21) In both of 1 w and 7 w mice, DNA-PKcs Ser2056 phosphorylation increased after γ-ray irradiation (Fig. 3). Again, 1 w mice exhibited higher levels of DNA-PKcs Ser2056 phosphorylation than 7 w mice at 6 hr after 4 Gy γ-irradiation (Fig. 3).

Expression and spatial distribution of DNA-PKcs in the brain tissues of mice examined by immunostaining. Panels a, b, and c show whole brains, cerebellum, and cerebrum, respectively. In panel a, black and white arrows indicate the cerebellum and cerebrum, respectively. Note that 4 sections on the left, i.e., those from 1-week-old (1 w) mice or 7-week-old (7 w) mice either irradiated or unirradiated, were on a single slide glass. As a negative control, the primary antibody, i.e., the anti-DNA-PKcs antibody, was omitted. In the top and bottom left panels of b, “E” and “I” indicate the external granular layer (EGL) and internal granular layer (IGL), respectively. All panels in “b” and “c” are shown at the same magnification, and the bar in the top left panel of “b” shows 100 µm. Experiments were repeated three times, with changing antibody concentrations, and representative results are displayed.

DNA-PKcs phosphorylation at Ser2056 and its spatial distribution in the brain tissues of mice examined by immunostaining. Panels a, b, and c show whole brains, cerebellum, and cerebrum, respectively. In panel a, black and white arrows indicate the cerebellum and cerebrum, respectively. Note that 4 sections in the left, i.e., those from 1-week-old (1 w) mice or 7-week-old (7 w) mice either irradiated or unirradiated, were on a single slide glass. As a negative control, the primary antibody, i.e., the anti-Ser2056-phosphorylated DNA-PKcs antibody, was omitted. In the top and bottom left panels of b, “E” and “I” indicate the external granular layer (EGL) and internal granular layer (IGL), respectively. All panels in “b” and “c” are shown at the same magnification and the bar in the top left panel of “b” shows 100 µm. Experiments were repeated three times, with changing antibody concentrations, and representative results are displayed.
It was also noted that, in the cerebella of 1 w mice, DNA-PKcs Ser2056 phosphorylation was pronounced in the internal granular layer (IGL) compared with the external granular layer (EGL) (Fig. 3b), although the expression of DNA-PKcs was apparently similar in both layers (Fig. 2b). Because cells in the EGL migrate toward the IGL, the EGL is diminished in the cerebella of 7 w mice, and only IGL was observed.
Differential DNA double-strand break repair kinetics in the brain of neonatal mice and young adult mice.We sought to compare the kinetics of DSB repair in the brain tissues of 1 w and 7 w mice using DSB markers, including γ-H2AX, i.e., Ser139-phosphorylated histone H2AX, and 53BP1. Because we found a considerable difference in the abundance of 53BP1 between the brains of 1 w and 7 w mice (much higher in the former than in the latter), we used γ-H2AX to examine the repair kinetics.22)
In the cerebella (Fig. 4) of 1 w and 7 w mice, γ-H2AX was enhanced strongly at 1 hr after irradiation. In 1 w mice, γ-H2AX declined considerably by 3 hr after irradiation (Fig. 4c) and mostly diminished by 6 hr after irradiation (Fig. 4d). On the other hand, in the cerebella of 7 w mice, γ-H2AX was retained even 6 hr after irradiation (Fig. 4j). This observation indicated that DSBs were repaired more rapidly in the cerebella of 1 w mice than in 7 w mice.

Spatio-temporal kinetics of DNA double-strand breaks in the cerebellum of mice examined by γ-H2AX immunostaining. Sections were stained with hematoxylin and anti-γ-H2AX antibody. Hematoxylin and eosin-stained sections are shown in Supplementary Fig. 1. Panels a to f show cerebella of 1-week-old (1 w) mice and panels g to l show the cerebella of 7-week-old (7 w) mice. a and g: unirradiated control; b and h: 1 hr after 4 Gy γ-irradiation; c and i: 3 hr after 4 Gy γ-irradiation; d and j: 6 hr after 4 Gy γ-irradiation; e and k: 12 hr after 4 Gy γ-irradiation; f and l: 24 hr after 4 Gy γ-irradiation. All panels are shown at the same magnification, and the bar in the bottom left of panel “a” shows 100 µm. In panels a and g, “E” and “I” indicate the external granular layer (EGL) and internal granular layer (IGL), respectively. Sections from three different mice were examined for each experimental group, giving similar results, and representative ones are displayed.
It is also noted that, in the cerebella of 1 w mice, γ-H2AX persisted longer in the EGL than in the IGL: more γ-H2AX-positive nuclei remained 3 hr after irradiation in the EGL than in the IGL (Fig. 4c). γ-H2AX increased at 12 and 24 hr after irradiation (Fig. 4e, 4f), probably reflecting DSBs arising in apoptosis.22) In conjunction with this, the thickness of the EGL was considerably decreased 24 hr after irradiation (Fig. 4f and Supplementary Fig. 1). We also examined the appearance of cleaved-caspase-3, which is commonly used as a marker for apoptosis.23) In 1 w mice, cleaved-caspase-3-positive cells were seen most evidently on the outer edge of the EGL (Supplementary Fig. 3c) and in the middle and/or inner part of the EGL at later time points (Supplementary Fig. 3d to 3f), in accordance with intensely γ-H2AX-stained nuclei. This observation also suggested that γ-H2AX-positive nuclei remaining in the EGL 3 hr after irradiation might represent cells undergoing apoptosis and, thus, most DSBs might have been repaired within 3 hr after irradiation in the EGL as well as the IGL. Cleaved-caspase-3-positive cells were not observed in the cerebella of 7 w mice at any time point (Supplementary Fig. 3g to 3l).
γ-H2AX kinetics in the cerebrum of 1 w mice were similar to that in the cerebellum (Fig. 5a to 5f, Supplementary Fig. 2). In the cerebrum of 7 w mice, γ-H2AX was observed even without irradiation (Fig. 5g) but increased 1 hr after 4 Gy irradiation (Fig. 5h). This observation suggested the presence of persistent or spontaneous DSBs in the cerebra of young adult mice even without irradiation.

Spatio-temporal kinetics of DNA double-strand breaks in the cerebrum of mice examined by γ-H2AX immunostaining. Sections were stained with hematoxylin and anti-γ-H2AX antibody. Hematoxylin and eosin-stained sections are shown in Supplementary Fig. 2. Panels a to f show the cerebra of 1-week-old (1 w) mice and panels g to l show the cerebra of 7-week-old (7 w) mice. a and g: unirradiated control; b and h: 1 hr after 4 Gy γ-irradiation; c and i: 3 hr after 4 Gy γ-irradiation; d and j: 6 hr after 4 Gy γ-irradiation; e and k: 12 hr after 4 Gy γ-irradiation; f and l: 24 hr after 4 Gy γ-irradiation. All panels are shown at the same magnification, and the bar in the bottom left of panel “a” shows 100 µm. Sections from three different mice were examined for each experimental group, giving similar results, and representative ones are displayed.
In this study, we demonstrated that DNA-PKcs is expressed at higher level in the brain tissues of neonatal (1 w) mice than in young adult (7 w) mice. Accordingly, DSBs were repaired more rapidly in the brains of neonatal mice than in the brains of young adult mice. In the study by Hudson et al., DSBs in the cerebellum and frontal cortex 30 min after 1 Gy irradiation modestly increased with age and were almost completely repaired 24 hr after irradiation in all age groups examined.12) In the current study, we examined DSBs at intermediate time points and found differences in the kinetics of DSB repair at around 3 and 6 hr after irradiation and also delayed DSBs, which were observed at 3 to 24 hr after irradiation, probably representing apoptosis.
A number of studies have demonstrated that NHEJ dysfunction causes neurological disorders, suggesting essential roles of NHEJ in neuronal development. Xrcc4−/− mice and Lig4−/− mice were embryonic lethal, showing massive apoptosis in the brain.24)–26) Ku70−/− or Ku80−/− mice also showed mild defects in neurogenesis.27) Although Prkdc−/− mice show no apparent defects in neurogenesis, they exhibit enhanced apoptosis of neuronal progenitors compared with control mice when exposed to ionizing radiation.28) It was also shown that mice expressing a catalytically inactive mutant of DNA-PKcs exhibited defective neurogenesis leading to embryonic lethality.29) A human individual with mutation in the Prkdc gene exhibited neurological disorders.30) Defective neurogenesis in NHEJ gene knockout mice can be rescued by homozygous or heterozygous loss of Tp53.31),32) However, Xrcc4−/−Tp53−/− or Lig4−/−Tp53−/− double knockout mice were shown to be highly prone to medulloblastoma.33),34) Brain tissues may suffer from a substantial amount of DNA damage, including base damage as well as single-strand breaks and DSBs, particularly in the developmental period, possibly due to high level of metabolism and/or transcription. Among such DNA damage, DSBs should be repaired by NHEJ or, if not repaired, activate apoptotic machinery in order to prevent carcinogenesis. If mice are irradiated during the developmental period, radiation-induced DSBs may interact with naturally occurring DSBs. For example, if these DSBs occur simultaneously in close vicinity, the probability of erroneous joining, such as deletion, inversion and translocation, will be increased, potentially elevating the risk for carcinogenesis. This might be underlying the medulloblastoma in the Ptch1 heterozygous background, which is induced within a narrow window of time centered on the days around birth.7)–9)
We also noted that, in contrast to DNA-PKcs, the expression of XLF was greatly reduced in 1 w mice compared with 7 w mice. It remains to be clarified this reduced amount of XLF is sufficient for DSB repair. In this regard, it has been reported that the disruption of XLF in combination with DNA-PKcs or PAXX resulted in synthetic lethality, indicating that XLF has functional overlap, at least partially, with DNA-PKcs and PAXX in DSB repair through NHEJ.35)–37) Therefore, the reduction in XLF function in 1 w mice might be compensated for by other molecules, such as DNA-PKcs or PAXX.
Recently, Kashiwagi et al. demonstrated higher expression of DNA-PKcs and DNA-PK enzyme activity in murine neural stem/progenitor cells and differentiated neuronal cells than in murine embryonic fibroblasts cultured in vitro.38) They also showed that murine neural stem/progenitor cells and differentiated neuronal cells had a higher ability to repair DSBs and higher induction of apoptosis upon exposure to X-rays than murine embryonic fibroblasts.38) Moreover, Shimada et al. demonstrated higher expression of NHEJ genes, including DNA-PKcs, Ku70, Ku86, and XRCC4, in human induced pluripotent stem cells and derived neural progenitor cells than in the original fibroblasts.39) XLF and LIG4 showed markedly higher expression in neural progenitor cells compared with fibroblasts and induced pluripotent stem cells.39) Thus, higher expression of DNA-PKcs in neonatal mice observed in this study may reflect the alertness and preparedness for DNA damage in the developing neuronal system, which may be also applicable to humans.
Finally, although we focused on NHEJ proteins in this study, HR may also be important for DSB repair in the development of the brain. In the above-mentioned study by Shimada et al., HR genes, such as BRCA1, NBS1, and RAD51C, were shown to be highly expressed in neural progenitor cells compared with induced pluripotent stem cells and the original fibroblasts.39) Nevertheless, NHEJ and HR seem to play mutually distinct roles in brain development. Orii et al. showed that Xrcc2−/− mice, which are HR-deficient, exhibited massive apoptosis in the early stages of embryonic development and in actively proliferating regions of the brain.40) On the other hand, in Lig4−/− mice, apoptotic cells were evident at later stages of embryonic development and in post-mitotic cells.40) Considering this, even in the brain of neonatal mice, HR might have substantial roles in DSB repair in actively proliferating regions such as the EGL in the cerebellum. Further studies will be required to examine the age-dependency of the expression levels and functions of HR proteins as well as NHEJ proteins in brain tissues.
We thank Yi Shang, Mari Ogawa, and Shinobu Hirano for assistance and Yoshiya Shimada and Mikio Shimada for advice and discussions. This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (24390290 and 15H02817 to Y.M.).
Edited by Takao SEKIYA, M.J.A.
Correspondence should be addressed: Y. Matsumoto, Laboratory for Advanced Nuclear Energy, Institute of Innovative Research, Tokyo Institute of Technology, N1-30 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan (e-mail: yoshim@lane.iir.titech.ac.jp).
Supplementary materialsSupplementary materials are available at https://doi.org/10.2183/pjab.96.014.
DNA-dependent protein kinase
DNA-PKcsDNA-dependent protein kinase catalytic subunit
DSBdouble-strand break
EGLexternal granular layer
HRhomologous recombination
IGLinternal granular layer
NHEJnon-homologous end joining
XLFXRCC4-like factor
XRCC4X-ray repair cross-complementing 4