2023 Volume 99 Issue 4 Pages 103-130
2023 Volume 99 Issue 4 Pages 103-130
Molecular clouds (MCs) in space are the birthplace of various molecular species. Chemical reactions occurring on the cryogenic surfaces of cosmic icy dust grains have been considered to play important roles in the formation of these species. Radical reactions are crucial because they often have low barriers and thus proceed even at low temperatures such as ∼10 K. Since the 2000s, laboratory experiments conducted under low-temperature, high-vacuum conditions that mimic MC environments have revealed the elementary physicochemical processes on icy dust grains. In this review, experiments conducted by our group in this context are explored, with a focus on radical reactions on the surface of icy dust analogues, leading to the formation of astronomically abundant molecules such as H2, H2O, H2CO, and CH3OH and deuterium fractionation processes. The development of highly sensitive, non-destructive methods for detecting adsorbates and their utilization for clarifying the behavior of free radicals on ice, which contribute to the formation of complex organic molecules, are also described.
Extremely cold and dense regions in interstellar media are known as molecular clouds (MCs), which comprise large amounts of gases and dust grains, where the dust-to-gas mass ratio is approximately 10−2.1),2) The temperature of MCs is low (10–100 K) because of the presence of dust grains, which shield the inner region of the MCs from the radiation from external stars. The gas density, which mostly corresponds to the abundant H2 molecules, is 103–105 cm−3, and more than 150 types of molecular species have been identified.3) These molecular species evolve from atoms even in such low-temperature environments through processes referred to as chemical evolution.4) In the gas phase, ion–molecule and radical–radical reactions can occur even at low temperatures because they are typically barrierless. However, gas-phase reactions alone cannot explain the large variety and abundance of the molecular species in MCs. Surface physicochemical processes on low-temperature dust surfaces are suggested to play key roles in chemical evolution.5),6)
A dust particle with a typical diameter of ∼0.1 µm has a silicate or carbonaceous core. In the MC environment, the dust core is typically enveloped by an ice mantle; this dust-particle/ice-mantle system is known as icy dust. The ice mantle predominantly comprises amorphous-phase water ice (amorphous solid water; ASW) and other molecules such as CO, CO2, NH3, CH4, H2CO, and CH3OH.7) These molecules are thought to form on dust surfaces via reactions between atoms and primordial molecules such as CO and are eventually released into the gas phase when the temperature of the dust increases. Therefore, the physicochemical processes important for the chemical evolution on icy dust are (i) adsorption of atoms and molecules, (ii) surface diffusion of these species, (iii) bimolecular reactions, and (iv) desorption of products. Dust surface reactions are advantageous over gas-phase reactions in certain contexts. Because of low temperatures, various species can accumulate on dust and encounter each other many times as compared to the gaseous phase collisions. Furthermore, when reactants stay at neighboring sites on dust, they interact with each other for a long time. In other words, the chances of encounters and subsequent reactions between reactants are greater in dust than in gas-phase reactions, where the collision rate between the highly abundant hydrogen is approximately once a year. Another important role of dust is to function as a third body to dissipate the excess energy of the surface reaction. For example, in the gas phase, the collision of two ground-state H atoms (the recombination reaction) does not yield H2 molecules because the heat of reaction (4.5 eV) cannot be released through the emission of a photon; in contrast, the recombination reaction occurs efficiently on dust surfaces.8) Because of their relevance to icy dust, the chemical processes occurring on ASW have been investigated in several laboratory and theoretical studies. Therefore, representative experiments on the physicochemical processes occurring on ASW are discussed in this short review, with a focus on our research.
Among the surface processes relevant to icy dust in MCs, the reactions of abundant H atoms are considered important; the accumulation rate of H atoms on dust is approximately once a day in MCs. Because of their low mass, physisorbed H atoms move efficiently on the surface even at 10 K; furthermore, tunneling reactions are expected to occur owing to the enhancement of the wave nature of H atoms at low temperatures. Laboratory investigations of H-atom reactions on ASW are introduced in Section 2. An important milestone has been reached because of laboratory experiments that clarified the formaldehyde (H2CO) and methanol (CH3OH) formation routes in an MC environment.9),10) As detailed in Section 2.3, several groups have demonstrated that these molecules are formed by successive hydrogenation of CO on the ASW surface; the addition of two H atoms to CO produces H2CO, and the subsequent addition of two H atoms yields CH3OH. Recently, certain H-atom reactions have been found to cause desorption of products. This process, known as reactive desorption or chemical desorption, is an important mechanism of desorption from dust without an external energy input such as photons and ion bombardments (Section 2.4).
Astronomical observations revealed that deuterated species are abundant in MCs.11) Although the abundance ratio of (partially) deuterated isotopologues to the normal isotopolog depends not only on species but also on MCs, it is a few-orders-of-magnitude higher than the abundance ratio of deuterium atoms to hydrogen atoms (D/H = 10−5) in interstellar media.12) This phenomenon, known as deuterium fractionation or enrichment, basically results from the greater thermodynamic stability of deuterated species than that of the corresponding normal species, and leads to the former being more prominent under lower-temperature conditions such as those in MCs. Deuterium enrichment has also long been recognized in meteorites and comets.13) The D/H ratio in terrestrial seawater is approximately one-order-of-magnitude higher than the interstellar abundance ratio. Because a similar degree of deuterium enrichment has been found for H2O molecules in comets,14) deuterium enrichment in the solar system is thought to originate at least partially from the MC that produced the Sun.15) In other words, deuterium fractionation proceeds significantly quickly in MCs at low temperatures, and it does not equilibrate at higher temperatures even after the formation of planetary systems. The extremely high deuterium fractionation in MCs cannot be explained by gas-phase mechanisms alone. Deuterium fractionation has been extensively investigated by our group, especially for formaldehyde and methanol, whose deuteration levels were found to be as high as 0.1 relative to the normal species in MCs (Section 3). We have demonstrated that efficient H–D substitution reactions (such as H2CO + H → HCO + H2 and HCO + D → HDCO) facilitate deuterium enrichment of molecules on icy dust. Our proposed H–D substitution mechanism has been incorporated into standard chemical models for deuterium enrichment in MCs.16)
The H-atom reactions alone cannot explain the formation of complex organic molecules (COMs) that have been observed in various astronomical objects; molecules comprising more than six atoms (excluding methanol) are often termed as COMs in the astrochemical community.17) Skeletal evolution in molecules via C–C bond formation, for instance, is necessary to form COMs. The reactions of radicals such as OH, HCO, CH3O, CH2OH, NH, and NH2 are believed to yield diverse COMs.18) These radicals must diffuse to meet each other on the surface of icy dust and ensure COMs formation; however, a non-diffusive mechanism, in which two radicals are produced in the vicinity, has also been proposed.19) Because these radicals are considerably heavier than the H atom, their diffusion is activated when the temperature of the dust and gas gradually increases from 10 to ∼200 K during star formation. Therefore, the temperature at which each radical begins to diffuse is an important parameter for understanding the formation of COMs. However, this parameter has not been experimentally determined because of the difficulty in in situ detection of radicals on ASW. To experimentally monitor the behavior of radicals on ASW, we developed a new method by combining photostimulated desorption and resonance-enhanced multiphoton ionization (PSD-REMPI) to sensitively detect trace amounts of surface adsorbates.20),21) The application of this method to OH radicals, which are thought to be abundant on ASW, is described in Section 4.1. The Cs+-ion pickup technique was also developed by our group as another sensitive method to study radical reactions on ASW. This method and its utilization for clarifying COM formation processes are detailed in Section 4.2.
Furthermore, our group has contributed experimentally to enhancing the knowledge on the other important physicochemical processes occurring on dust surfaces. Relevant aspects such as the ortho-to-para conversion of H2 on dust grain surfaces, the ortho-to-para ratio of water molecules desorbed from ASW, and the shape of icy grains are briefly discussed in Section 5. Future perspectives are presented in Section 6.
Molecular hydrogen (H2) is the most abundant molecule in the Universe and plays important roles in several astronomical processes, including chemical reactions and gas dynamics during star formation. Thus, the mechanisms of H2 formation in interstellar environments are a crucial topic in astrochemical and astrophysical research communities.5),8),22),23) The gas-phase formation of H2 is inefficient because of the forbidden radiative association reaction between H atoms in the ground state (H + H → H2 + hν), and the three-body reaction (H + H + M → H2 + M) is unfeasible under the ultrahigh-vacuum conditions of MCs; moreover, the electron-based reactions (H + e− → H− + hν, H− + H → H2 + e−) are remarkably slow because of the scarcity of electrons.24) Consequently, other H2 formation processes should be considered to account for the observed high abundance in interstellar media. Generally, H2 is thought to be produced on dust grain surfaces,25) which permit interactions between H atoms and act as a third body “M” to dissipate the heat of reaction (4.5 eV). Because the recombination reaction itself is barrierless, the rate of H2 formation on the grain surfaces is dominated by the surface diffusion rate of H atoms. Thus, to elucidate the H2 formation mechanisms in MCs, investigations of H-atom diffusion on low-temperature surfaces, particularly on ASW, are critical.
Most experimental studies on the H-atom diffusion on ASW have employed temperature-programmed desorption (TPD), in which the H2 molecules desorbing from the H-atom-deposited ASW during a temperature increase are detected using a quadrupole mass spectrometer (QMS). The H-atom diffusion is indirectly deduced from TPD spectra, which are plots of the H2 desorption rate as a function of the surface temperature. However, the TPD method can cause a large ambiguity because the TPD spectral analysis involves several parameters such as the number density, desorption rate, and surface diffusion rate to derive the activation energies for H2 desorption (Edes) and H-atom diffusion (Esd) and desorption. Furthermore, TPD is unsuitable for analyzing irregular amorphous surfaces, where the activation energy cannot be represented by a single value but has a wide distribution, and often provides contradictory results. For example, Perets et al. and Matar et al. reported differing Esd values of H atoms (40–55 and 22 ± 2 meV, respectively)26),27); the former suggests that H(D) atoms hardly diffuse at 10 K, whereas the latter indicates that they can diffuse on ASW within the experimental timescale. If the H atoms and H2 molecules on the surface can be detected without employing TPD, the Esd of H atoms can be determined more unambiguously. To that end, we developed a new experimental method known as PSD-REMPI.20),21)
In the experimental setup used for the PSD-REMPI measurements of H atoms and H2 molecules on ASW (Fig. 1a), an aluminum (Al) substrate is located at the center of an ultrahigh-vacuum chamber; the temperature of the substrate can be controlled between 6 and 300 K using a closed-cycle He refrigerator and a heater. To prepare the ASW sample, H2O vapor is introduced into the chamber through a capillary plate and deposited onto the substrate maintained at 8 K. Subsequently, hydrogen atoms produced by a microwave discharge created in a Pyrex tube are introduced into the main chamber through an Al pipe at 100 K to reduce the translational energy. The scheme governing the PSD-REMPI detection is illustrated in Fig. 1b. Essentially, the hydrogen atoms on the ASW surface are photodesorbed using a PSD laser (532 nm, 10 ns duration, <100 µJ per pulse). Although the mechanism of the PSD is unclear, the H-atom desorption presumably results from the propagation of phonons produced on the Al substrate.28) Then, the desorbed H atoms are ionized using a REMPI laser. The (2 + 1) REMPI scheme involving the 2s ← 1s transition was used to ionize H atoms. A linear time-of-flight (TOF) mass spectrometer was used to detect the ionized H atoms (that is, H+ ions). As shown in Fig. 1c, the intensities of the PSD-REMPI signal for H atoms increases linearly with deposition time. Because the H-atom flux is constant and low enough to assume that the surface number density of H atoms (nH) increases linearly with the deposition time, the PSD-REMPI intensities are considered to be proportional to nH.29)

(a) Schematic of experimental apparatus used for PSD-REMPI-based detection of H atoms. (b) Timing chart and scheme adopted for the H-atom detection. (c) Increase in H atoms photodesorbed from ASW during the deposition of H atoms at 8 K.29) (d) Attenuation of H-atom intensity as a function of the waiting time after deposition; data related to H atoms on ASW at 8 K, ASW at 12 K, and PCI at 8 K are shown as filled circles, a filled triangle, and an open circle, respectively.28)
After the deposition of H atoms on the ASW surface, nH is expected to decrease with time (waiting time in Fig. 1b) via monoatomic desorption and/or recombination reactions to form H2. Thus, the rate equation for the time variation of nH is expressed as
| \begin{equation} \frac{dn_{\text{H}}}{dt} = - 2k_{\text{H-H}}n_{\text{H}}^{2} - k_{\text{des}}n_{\text{H}}, \end{equation} | [1] |
| \begin{equation} \frac{I_{\text{H}}}{I_{0}} \propto \frac{n_{\text{H}}}{n_{0}} = \frac{1 - b}{2k_{\text{H-H}}n_{0}t + 1} + b, \end{equation} | [2] |
| \begin{equation} 2k_{\text{H-H}}n_{0} = \theta \nu \exp \left(- \frac{E_{\text{sd}}}{k_{\text{B}}T} \right), \end{equation} | [3] |
As described above, the surface diffusion of H(D) atoms on ASW occurs through a thermal hopping mechanism. However, the diffusion of H atoms on polycrystalline ice (PCI) occurs remarkably quickly, hindering the scrutiny of nH by PSD-REMPI with varying waiting times after the H-atom deposition.28) This quick decline of H atoms on PCI suggests the fast diffusion through the tunneling effect. Another interesting issue regarding H-atom diffusion is the viability of tunneling diffusion. Kuwahata et al. employed another methodology to assess the diffusion on PCI for evaluating the diffusion-related kinetic isotope effect.30) They monitored the intensities of H or D atoms on PCI under steady-state conditions during continuous deposition of H or D atoms with identical fluxes on PCI at 10 K; the steady-state condition refers to a balance between the supply and loss of H (D) atoms (ps·F = kH-HnH2, where ps and F are the sticking coefficient, which is assumed to be the same for H and D atoms, and atomic flux, respectively). For identical fluxes of H and D atoms in each experiment, the ratio of the surface number densities for H and D atoms can be expressed simply using the recombination rates kH-H and kD-D:
| \begin{equation} \frac{n_{\text{D}}}{n_{\text{H}}} = \sqrt{\frac{k_{\text{H-H}}}{k_{\text{D-D}}}}. \end{equation} | [4] |
Because the surface composition of icy dust grains is considered to partly include solid CO, H-atom diffusion on pure solid CO—which is the second most abundant molecule after H2—was investigated by our group.34) By analyzing the attenuation curve of the PSD-REMPI intensities, Kimura et al. determined the Esd values for H atoms on solid CO to be ∼22, 30, and 37 meV at surface temperatures of 8, 12, and 15 K, respectively. These Esd data are representative values at the corresponding temperatures because the surface of the solid CO sample produced by vapor deposition at 10 K is typically rough and has several types of sites distinguished with adsorption potential depths; at higher temperatures, the diffusion is dominated by the activation energy for deeper adsorption sites.
MCs evolve from diffuse clouds in which the temperature is relatively high (∼80 K) and grains are not covered with ice. H2 is abundantly produced in this environment; however, the related mechanism is unclear. Therefore, we studied the H2 formation processes on diamond-like carbon (DLC), which serves as a model surface for bare grains (that is, ice-free grains).35) An analysis similar to that performed by Kuwahata et al.30) revealed the significance of tunneling diffusion in the diffusion of H atoms on the DLC surface. Moreover, we found that H2 was formed efficiently on the DLC even at 20 K, which is higher than the threshold for H2 formation on ASW. However, the formation at 20 K is feasible only under laboratory conditions of high H-atom fluxes; therefore, other mechanisms that are responsible for H2 formation in diffuse clouds must be clarified.
2.2. H2O formation.The abundance of H2O in space cannot be explained only by the gas-phase route H3O+ + e− → H2O + H36); the surface reactions on dust in MCs, as originally proposed by Tielens and Hagen,37) should also be considered. In fact, H2O is the dominant component of the icy mantle. Several mechanisms have been proposed for H2O formation on dust surfaces based on laboratory experiments, as detailed in a review article.23) The simplest mechanism involves reactions featuring the addition of H-atoms to O atoms ($\text{O}\overset{\text{H}}{\to}\text{OH}\overset{\text{H}}{\to}\text{H$_{2}$O}$). As both steps are barrierless radical–radical reactions, the rate of H2O formation is determined by the surface diffusion of H atoms. Another important H2O-formation process is the successive addition of H atoms to O2 molecules38):
| \begin{equation} \text{O$_{2}$}\overset{\text{H}}{\to}\text{HO$_{2}$}\overset{\text{H}}{\to}\text{H$_{2}$O$_{2}$}\overset{\text{H}}{\to}\text{H$_{2}$O} + \text{OH}. \end{equation} | [5] |
Miyauchi et al. performed experiments using an apparatus similar to that shown in Fig. 1a.41) In their experiments, reflection–absorption infrared (IR) spectrometry was used to trace the evolution of a solid O2 sample subjected to H-atom irradiation. The difference IR spectra collected after H-atom irradiation of a solid O2 sample at 10 K (Fig. 2) helped verify the formation of H2O2 and H2O. Interestingly, the shape of the OH-stretching feature in the IR spectrum, which is expected in the region 3000–3700 cm−1, indicated that the solid H2O formed by this reaction was not crystalline but amorphous, which is consistent with the characteristics of abundant ASW in the icy mantle of dust grains. Similar experiments were performed using D atoms, and the formation of D2O2 and D2O was confirmed. The formation rates of H2O2 and D2O2 were similar, as expected for barrierless reactions. However, the rate of H2O formation was one-order-of-magnitude higher than that of D2O, indicating that the last step in Reaction [5] occurred via quantum mechanical tunneling.

Difference IR spectra acquired upon H-atom irradiation of solid O2 at 10 K.41) Positive features indicate production. The IR features of H2O2 and H2O are indicated.
The formation of H2O molecules upon the co-deposition of O2 molecules and H atoms was investigated by Oba et al.42) Continuous formation of H2O and H2O2 was observed even at 40 K. A particularly important finding was that amorphous H2O ice formed was compact (nonporous), as indicated by the absence of dangling OH features in the IR spectra, which is consistent with astronomical observations of MCs.43) However, the exact morphology of ASW in space—compact or otherwise—is unclear, because the dangling OH feature was tentatively identified in a recent observation of a dense region of an MC.44)
Another H2O-formation process involving the reaction between H2 and OH radicals (H2 + OH → H + H2O) was examined by Oba et al.45) This reaction has a barrier of ∼180 meV in the gas phase,46) indicating that the thermal reaction does not occur at low temperatures such as 10 K. Using several combinations of reactants (H2, D2, HD, OH, and OD), the authors experimentally confirmed the occurrence of this reaction. Moreover, the product yield in the H-atom abstraction reaction (HD + OH → D + H2O) was one-order-of-magnitude higher than that in the D-atom abstraction reaction (HD + OH → H + HDO). This difference was explained by considering the fact that the effective mass for quantum mechanical tunneling in the former case was approximately half that in the latter case.
2.3. Successive hydrogenation of CO.Simple organic molecules—H2CO and CH3OH—are abundantly present not only in the gas phase, but also in icy dust and some comets. These molecules appear to have evolved from CO molecules on dust through successive hydrogenation (that is, H-atom addition), photolysis, and/or cosmic-ray proton bombardment on H2O–CO ice. Because of the ubiquity of these molecules in various astronomical objects and their importance as precursors of COMs, the origin of these molecules has been intensively studied. Successive hydrogenation, expressed as follows:
| \begin{equation} \text{CO}\overset{\text{H}}{\to}\text{HCO}\overset{\text{H}}{\to}\text{H$_{2}$CO}\overset{\text{H}}{\to}\text{CH$_{3}$O/CH$_{2}$OH}\overset{\text{H}}{\to}\text{CH$_{3}$OH}, \end{equation} | [6] |
Subsequently, several types of experiments targeting Reaction [6] on ASW were performed in the 2000s, as elaborated in a review.5) The total reaction rate is one-order-of-magnitude smaller in the experiments conducted using D atoms, presumably because the first and third steps in Reaction [6] have barriers and proceed via quantum mechanical tunneling at low temperatures.50) The conversion rate of the reaction H2CO → CH3OH at 15 K was found to be approximately half that for CO → H2CO.51) The yield of hydrogenation reactions depends significantly on the surface composition (solid CO, CO on ASW, or H2O–CO ice) and surface temperature, which affect the sticking coefficient of H atoms and their residence time on the surface.52)–54)
Based on the aforementioned experimental findings, successive hydrogenation of CO on ASW is thought to occur only at temperatures below 20 K, where H atoms can remain on the surface. Recently, we found that when CO molecules are buried deep in ASW, the successive hydrogenation reactions occur even at 70 K because certain H atoms that collide with the ASW surface can penetrate the ASW along the walls of cracks and may have a prolonged residence time.55) The penetration depths of H atoms for porous and nonporous ASW were determined to be >50 and ∼10 monolayers, respectively,56) where one monolayer of ASW has a thickness of approximately 0.3 nm. This type of reaction is important for any atom or molecule embedded in ASW.21)
2.4. Chemical desorption.Similar to the aforementioned discussion on the efficient formation of formaldehyde and methanol on ice, diverse COMs have been experimentally confirmed to be produced on ice surfaces.17),57) Certain gas-phase COMs have been detected using radio telescopes even in cold MCs.58) Desorption should occur when these species are produced on ice and detected in the gas phase. Thermal desorption is negligible at temperatures as low as 10 K, and photodesorption is inefficient because of the extremely low flux of UV radiation in the dense MC region. Therefore, an additional desorption mechanism—reactive desorption or chemical desorption—has been proposed.59) Therein, a reaction product desorbs because of the heat of reaction. Although the heat of reaction is generally much larger than the adsorption energy in the physisorption case, the desorption of products following their formation is not obvious. In fact, during the hydrogenation of CO (Reaction [6]), most of the products of H2CO and CH3OH remain on the surface, implying that a significant fraction of the excess energy dissipates into the bulk surface. Although the exact dynamics of chemical desorption remain unclear, the occurrence of chemical desorption has been demonstrated in several laboratory experiments.60)–64) Nevertheless, the efficiency of chemical desorption should depend on the reactions, and it is often included in astrochemical models through an equation that relates the probability of chemical desorption and the excess energy of reaction.65)–68)
In 2018, Oba et al. analyzed the chemical desorption of H2S through in situ IR measurements.62) They investigated chemical desorption during the reactions H2S + H → HS + H2 and/or HS + H → H2S through IR measurements for the first time. The occurrence of these two reactions was confirmed by monitoring the formation of HDS and D2S upon exposing H2S to D atoms. As shown in Fig. 3, the amount of H2S adsorbed on ASW significantly decreased upon exposure to H atoms at 10 K. Because the parent and final product molecules obtained via the exposure of H2S to H atoms were both H2S as mentioned above, the decrease was presumably due to desorption of products from the surface. The exothermicity values of the reactions H + H2S → H2 + HS and HS + H → H2S are ∼0.6 and 3.8 eV, respectively, and the binding energies calculated for HS and H2S on a water cluster are both ∼0.1 eV. Based on exothermicity, Oba et al. deduced that chemical desorption is more likely to occur upon the formation of H2S. They estimated the effective cross-section for chemical desorption and found that the chemical desorption rate exceeded the expected photodesorption rate in a typical interstellar environment. Furthermore, the effective desorption cross-section is not strongly influenced by the ice structure (porous amorphous, nonporous amorphous, or crystalline) and temperature (10–30 K).63) Recently, Nguyen et al. found that phosphine molecules (PH3) on ice surfaces can also be desorbed into the gas phase via chemical desorption through the reactions H + PH3 → H2 + PH2 and/or PH2 + H → PH3.64)
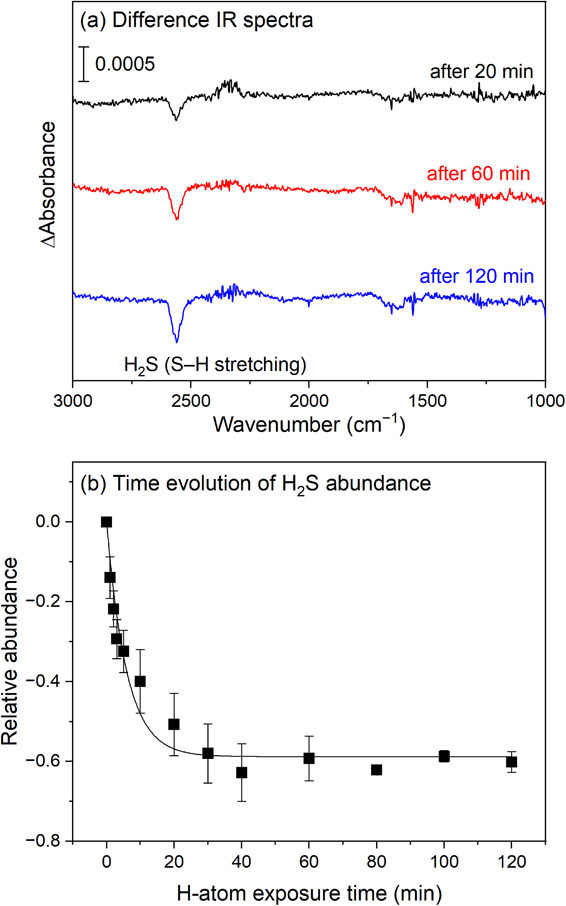
(Color online) (a) Difference IR spectra collected after H-atom irradiation of H2S adsorbed onto ASW at 10 K. The S–H stretching intensity decreases near 2560 cm−1. (b) Attenuation curve for H2S adsorbed onto ASW during the H-atom irradiation. The solid line represents a single-exponential fit to the data (H-atom flux = 1.3 × 1014 atoms cm−2 s−1). These figures were adopted from Ref. 62.
The aforementioned experimental results indicate that the chemical desorption of H2S and PH3 can potentially affect the chemistry of sulfur and phosphorus in interstellar media. Furuya et al. attempted to constrain the desorption probability of H2S and PH3 per reaction event on ASW by numerically simulating laboratory experiments.69) The desorption probabilities for H2S and PH3, which are often assumed to be 1% in chemical models, were estimated to be (3 ± 1.5)% and (4 ± 2)%, respectively, using the microscopic kinetic Monte Carlo method.
2.5. Other reactions.To acquire further insight into the chemistry of sulfur and phosphorus in MCs, we extended our investigations to H-atom reactions with relevant species. Carbonyl sulfide (OCS) is an abundant S-bearing molecule in MCs that has been found both in the gas phase and on icy grains.70),71) Thus, the OCS molecule can potentially react with H atoms on icy grains. Three possible pathways exist for hydrogen addition to OCS: cis-HOCS (addition to O), OC(H)S (addition to C), and trans-OCSH (addition to S). The three reactions have relatively large barriers, as evidenced by the values estimated for isolated systems via quantum calculations performed at the CCSD(T)/aug-cc-pVTZ level72) (25.2, 40.4, and 93.4 kJ mol−1, respectively). If the shape of the barrier in these reactions is assumed to be similar, the H-atom tunneling reaction preferentially yields cis-HOCS. Nguyen et al. performed co-deposition experiments of OCS and H atoms.73) They did not observe any mono-hydrogenated OCS, but obtained CO, hydrogen sulfide (H2S), H2CO, and CH3OH as the main products, with a minor yield of thioformic acid (HC(O)SH). Quantum chemical calculations were performed for the successive hydrogenation of OCS on ASW. Similar to the calculations performed on the isolated system, the addition of H to S, yielding OCSH, has the lowest barrier of 0.21 eV (20.3 kJ mol−1) for the first reaction (OCS + H);73) the initial product trans-OCSH readily isomerizes to cis-OSCH owing to the exothermicity of the H-addition reaction.72) A secondary reaction between OSCH and an H atom yields HOCSH, OC(H)SH, and a CO–H2S pair, with the last channel being most favorable energetically. Thus, calculations have suggested that CO and H2S are the main products and OC(H)SH is a minor product, which is consistent with the experimental results, whereas H2CO and CH3OH are thought to be produced via successive hydrogenation of the CO product.
The reactions between PH3 molecules and H(D) atoms on ASW have been studied both experimentally and theoretically.74) The H–D substitution reaction of PH3 and the D–H substitution reaction of PD3 occurred upon D- and H-atom irradiation, respectively, at 10 K on ice surfaces. Although the H- and D-abstraction reactions with a barrier height of ∼0.13 eV likely occurred via quantum mechanical tunneling, the H–D substitution reaction of PH3 was slightly slower than the D–H substitution reaction of PD3 under the employed experimental conditions. These results indicate that the relative rates of the H–D and D–H substitution reactions were dictated not by the kinetic isotope effect in the tunneling, but by other elementary processes such as atomic diffusion on ice. Based on their experimental observations, the authors speculated the presence of a deuterated phosphine isotopolog (PH2D) in PH3-rich interstellar environments. This topic (that is, H-atom reactions with OCS and PH3) has been reviewed from the viewpoint of theoretical calculations.75)
The isomerism of molecules and their mechanisms in the interstellar environment are unresolved questions in the chemistry of organic molecules in space.76) Formic acid (HC(O)OH) is the simplest organic acid exhibiting cis–trans conformational isomerism.77),78) Formic acid is mostly (>99.9%) in its trans conformation under ambient conditions because of its thermodynamic stability, whereas abundant cis-formic acid has been identified in the interstellar photodissociation regions (up to 33%) and cold cores (6%).79),80) Molpeceres et al. attempted to explain the abundance of the cis-form based on the hydrogen abstraction reactions.76) Theoretical calculations revealed a clear dependence of the H-abstraction reactions on the isomer of the reactant, with the rate constants for the cis-isomer at ∼50 K being five-orders-of-magnitude greater than those of the trans-isomer. These results indicated that the H-abstraction reactions reduced the abundance of the cis-form, in contrast to significant presence of cis-form found by astronomical observations. To confirm these theoretical predictions, co-deposition experiments were performed using trans-formic acid and D atoms at 10 K. C–D stretching features were not observed in the IR spectra, indicating that the reaction trans-HC(O)OH + D → cis-HOCO + HD did not occur.76) Although this result is consistent with theoretical predictions, the reaction cis-HC(O)OH + D → trans-HOCO + HD was not investigated because they do not have the means to prepare cis-formic acid. Therefore, additional experiments based on IR-laser-induced trans-to-cis isomerization81) should be conducted to study the isomer-dependent reactivity in the H-abstraction reaction. Nevertheless, other mechanisms such as those leading to the preferential formation of the cis-form over the trans-form should be clarified in the future to explain the astronomically observed abundance.
Hydrogen reactions with aromatic molecules, particularly polycyclic aromatic hydrocarbons (PAHs), are considered important for their relevance to H2 formation.56),82)–84) Compared to the extensive investigations on gas-phase reactions, limited experimental studies have been conducted on the reaction between hydrogen and PAHs on ice. Hydrogenation reactions in solid benzene (C6H6) were studied by Hama et al.,85) who found that the reactivity depended significantly on the crystallinity (amorphous or crystalline) of the sample. The considerably higher reactivity in the amorphous solid was attributed to the difference in geometric constraints between the amorphous and crystalline surfaces, where the reactive benzene, that is “dangling benzene”, exists in considerably larger numbers on the amorphous surface. Although the H-atom addition reactions were found to proceed via quantum mechanical tunneling, a large kinetic isotope effect—characteristic of the tunneling reaction—was not observed.86) The significant reduction in the kinetic isotope effect was due to the control of chemical kinetics by the surface diffusion of the H/D atoms, which proceeds via a thermal hopping mechanism with a small kinetic isotope effect.
From astronomical observations, high D/H ratios (abundance ratios of deuterated molecules and their equivalents having only hydrogen) have often been observed in interstellar molecules, particularly in H2O, NH3, H2CO, and CH3OH. In the case of CH3OH, the abundance of its singly deuterated isotopologue—CH2DOH—in the star-forming region IRAS 16293-2422, has been reported to be ∼30% of the total CH3OH content.87) Moreover, even its triply deuterated isotopologue—CD3OH—has been detected in the same region. The formation of multiple deuterated molecules cannot be explained by ion–molecule reactions in the gas phase; therefore, the reactions on icy grains are likely responsible for the significant deuterium enrichment in CH3OH.
As described in Section 2.3, the formaldehyde and methanol in MCs are predominantly formed via successive hydrogenation of CO (Reaction [6]). Nagaoka et al.88) speculated that the reactions of D atoms during the formation of formaldehyde and methanol on dust play a role in the deuterium enrichment; they experimentally observed deuterium-enriched methanol upon exposing solid CO to H and D atoms simultaneously. Analyzing the time variation of the products of formaldehyde, methanol, and their deuterated isotopologues, the authors found that the H–D substitution reactions in methanol and formaldehyde are key routes for deuterium enrichment (Section 3.1.5).88) Figure 4 shows the network of reactions with H and D atoms in the CO–formaldehyde–methanol system. The network includes H (D) addition and H–D (D–H) substitution reactions on the reactant species. We experimentally examined each pathway in the network, as follows, to determine the process most responsible for deuterium enrichment in methanol:

(Color online) Surface reaction diagram for carbon monoxide (CO), formaldehyde (H2CO), and methanol (CH3OH) on icy dust.5) Compounds written in roman and italic type denote experimentally detected and undetected products, respectively. Species within square frames have been observed in MCs. Solid and dashed arrows represent reactions of H and D atoms, respectively.
When solid CO was irradiated with D atoms, fully deuterated formaldehyde (D2CO) and methanol (CD3OD) were formed, validating the successive addition of D atoms to CO molecules.50) As expected for tunneling reactions, the effective reaction rate for CO + D → DCO was one-order-of-magnitude smaller than that for CO + H → HCO, indicating that the addition of D to CO was not responsible for deuterium fractionation.
3.1.2. H2CO + D.In this experiment, H2CO was deposited onto ASW and subsequently irradiated with D atoms.89) Immediately after the D-atom irradiation was initiated, the H2CO content decreased and HDCO was formed, followed by the production of D2CO. This indicates that the H atoms in H2CO were replaced with D atoms, that is, the occurrence of H–D substitution reactions. Two H–D substitution reaction mechanisms are possible in this regard: sequential tunneling H-abstraction (H2CO + D → HCO + HD) and D-addition (HCO + D → HDCO), and direct H–D exchange (H2CO + D → HDCO + H). The sequential and direct mechanisms may compete with each other because of their similar activation energies (1899 and 1799 K, respectively, in the gas phase90)). Longer D-atom irradiation did not yield deuterated methanol isotopologues (CH2DOD, CHD2OD, or CD3OD), but produced D2CO. These results suggest that H–D substitution reactions occur preferentially over D-addition reactions. If the D–H substitution reactions, which reduce the degree of deuterium fractionation, are slow, the observed H–D substitution reaction in H2CO may involve deuterium fractionation of the formaldehyde in MCs.
3.1.3. D2CO + H.H-atom irradiation experiments were performed for D2CO deposited on ASW.89) After the H-atom irradiation, CHD2OH, HDCO, H2CO, and CH3OH were generated, with CHD2OH being the major product. The formation of HDCO and H2CO occurred via H–D substitution, and the H2CO product was further hydrogenated to yield CH3OH. After prolonged H-atom irradiation, the fluence of which corresponded to that in MCs for 106 years, CHD2OH and CH3OH were obtained in similar yields. A clear difference was observed between the H2CO + D and D2CO + H reactions; only the substitution reaction occurred in the former system, and both substitution and addition reactions occurred in the latter system.
3.1.4. CH3OH + D.CH2DOH, CHD2OH, and CD3OH were formed upon the D-atom irradiation of a solid CH3OH sample at 10 K.91) The time evolutions of CH2DOH, CHD2OH, and CD3OH indicated that they were produced through a sequence of substitution reactions:
| \begin{equation} \text{CH$_{3}$OH}\overset{k_{1}}{\to}\text{CH$_{2}$DOH}\overset{k_{2}}{\to}\text{CHD$_{2}$OH}\overset{k_{3}}{\to}\text{CD$_{3}$OH}, \end{equation} | [7] |
CH3OH-dn solids (n = 1–4), representing deuterated methanol with n deuterium atoms, were irradiated with H.91) However, D–H substitution reactions were not observed in any scenario, indicating that after the CH3OH-dn compounds were produced on ice, they did not transform into CH3OH through surface reactions with H atoms.
Experiments were performed using CH3OH isotopologues (CH3OH, CH2DOH, and CHD2OH) and D atoms to verify the relative rate of Reaction [7]; this parameter was estimated by simply fitting the time-decay curves acquired upon irradiating the isotopologues with D-atoms to a single-exponential function. The relative rates (k1/k2/k3) were determined to be 1/∼0.5/∼0.3. These results indicate that the relative reaction rates are effectively scaled by the number of H atoms in the methyl group, that is, the probability of a reacting D atom locating the H atom is highest in CH3OH and lowest in CHD2OH. Moreover, small differences in the C–H bond strengths of these isotopologues can affect the relative rate.
3.1.6. CO + H(D) reactions and comparison with astronomical observations.The aforementioned experimental results clearly demonstrate that the deuterium fractionation in formaldehyde and methanol proceeds via H–D substitution reactions, and that the deuterium addition reactions in CO and D–H substitution reactions in CH3OH-dn are inefficient. To estimate the abundance of H2CO-dn and CH3OH-dn on dust grains in MCs, Nagaoka et al.88) performed simulation experiments in which a solid CO sample was irradiated simultaneously with H and D atoms having a flux ratio (D/H) of ∼0.1, which was adopted from theoretical models. Various deuterated products—HDCO, D2CO, CH2DOH, CHD2OH, and CD3OH—were formed during the H- and D-atom irradiation.
The deposition of atoms with fluences corresponding to those expected in MCs for 1–2 × 106 years yielded the following H2CO-dn/H2CO and CH3OH-dn/CH3OH ratios (astronomically observed values corresponding to the low-mass protostar IRAS 16293-242287),93) are shown in parentheses): HDCO/H2CO = 0.1–0.5 (0.13–0.16), D2CO/H2CO = 0.03–0.06 (0.05–0.06), CH2DOH/CH3OH = 0.1–0.3 (0.3), CHD2OH/CH3OH = 0.15–0.3 (0.06), and CD3OH/CH3OH = 0.02–0.1 (0.01). These species are considered to have formed on icy grains sublimated by heat from the protostar. Because this protostar is relatively young (104 years old), the aforementioned species have not been subjected to gas-phase processes. Therefore, the observed H2CO-dn/H2CO and CH3OH-dn/CH3OH ratios are representative of those achieved through reactions on icy grain surfaces.94) A comparison between the laboratory simulation and observations indicates that the deuterium fractionation in formaldehyde and methanol was adequately reproduced in the simulation experiment. In particular, a high abundance of multiple deuterated species was observed experimentally. This level of abundance has not been achieved in chemical models that do not include the H–D substitution reactions. The experimental findings on the H–D substitution reactions have deepened our understanding of deuterium fractionation.
3.2. Other molecules. 3.2.1. Small molecules.D-atom irradiation experiments have also been performed on solid H2O, NH3, and CH4, which are deuterium-enriched interstellar molecules. Although these experiments were preliminary, we did not observe the H–D substitution reactions at 10 K. These results indicate that deuterated isotopologues of the aforementioned species can be formed only during production, for example, via OH + D → HDO and/or gas-phase reactions. Consequently, the degree of deuterium fractionation in these species is expected to be lower than those of H2CO and CH3OH. According to astronomical observations, the deuterium fractionation degree in H2CO and CH3OH is approximately one-order-of-magnitude larger than that of H2O and CH4.
The absence of H–D substitution reactions in these species can be attributed to reaction thermicity. The H–D substitution reaction is considered a two-step reaction, for example, CH4 + H → CH3 + H2 and CH3 + D → CH3D. According to quantum chemical calculations, the reaction CH4 + H(D) → CH3 + H2 (HD) is endothermic (ΔH ∼ 3 kJ mol−1) and has an activation barrier (Ea) of ∼60 kJ mol−1.92) This reaction cannot occur at low temperatures such as 10 K, even through quantum mechanical tunneling. However, the ΔH and Ea values for the reaction CH3OH + H → CH2OH + H2, whose occurrence at 10 K has been verified (Section 3.1), are reported to be −33 and 36 kJ mol−1, respectively. The H–D substitution reactions in other astrochemically relevant molecules were investigated by our group, and the results are briefly discussed in the following subsections.
3.2.2. Ethanol.Ethanol (CH3CH2OH) is a highly abundant COM found in star-forming regions. Although deuterated ethanol has not been astronomically detected, its production is believed to occur predominantly through dust-surface reactions. Therefore, Oba et al. performed D(H)-atom irradiation experiments on solid ethanol at 10 K.95) H–D substitution reactions were found to occur on the ethyl (CH3CH2) group but not on the hydroxyl (OH) group. When the deuterated ethanol solid was irradiated with H atoms, the D–H substitution reactions occurred only on the ethyl group. These results are consistent with those of methanol, where only the hydrogen (deuterium) atoms in the methyl group undergo substitution reactions. The rates of H–D and D–H substation reactions were found to be similar to each other whereas the reaction involves quantum mechanical tunneling. They considered that other elementary processes such as atomic diffusion on ice dominate the reaction rates. The deuterium fractionation of dimethyl ether (CH3OCH3) subjected to H–D substitution has also been reported.96) In the case of dimethyl ether, Oba et al. found that the H–D substitution rates for pure solid CH3OCH3 were 20 times greater than that for the CH3OCH3–H2O complex deposited on water ice, indicating a “negative” catalytic effect of water in the H–D substitution reactions. Based on experimentally determined reaction rates, the authors estimated the abundance ratios for CH3CHDOH/CH3CH2OH and CH2DCH2OH/CH3CH2OH after 106 years under MC conditions to be ∼1 × 10−2 and ∼6 × 10−3, respectively, and predicted the existence of deuterated ethanol as well as deuterated dimethyl ether in space.95)
3.2.3. Methylamine.Methylamine (CH3NH2) is considered one of the simplest building blocks of biomolecules in interstellar media and has been found in the comet Wild 2.97) This species would be formed both in the gas phase and on interstellar grains. Regardless, methylamine can be presumed to exist on the surface of icy grains at low temperatures (∼10 K) and undergo several physicochemical processes, including H(D)-atom reactions. Extensive laboratory simulation experiments have been conducted on this molecule under astrophysical conditions, and the formation of glycine—the simplest amino acid (NH2CH2COOH)—from methylamine has been reported.98),99) However, less attention has been paid to the deuterium fractionation in this molecule. If methylamine serves as a building block for amino acids or other biomolecules, its deuterium enrichment may propagate to its products. Therefore, Oba et al. investigated H–D substitution reactions.100)
After the exposure of solid CH3NH2 to D atoms, H–D substitution reactions were confirmed to occur on both the methyl (CH3) and amino (NH2) groups, which yielded CD3ND2 as the terminal product. In the experiments involving exposure of CD3ND2 to H atoms, CH3NH2 was generated as the product. Experiments on partially deuterated isotopologues (CH3ND2 or CD3NH2) revealed that the H–D (D–H) substitution reactions on the methyl group were faster than those on the amino group. The rates of D–H substitution reactions were found to be slower than H–D substitution reactions, indicating that H(D)-abstraction step via quantum mechanical tunneling is the rate-determining step. According to quantum chemical calculations, the H–D substitution in methylamine occurs either via an abstraction–addition mechanism or through the formation of an intermediate such as [CH3NH2D], followed by a reaction with another H(D) atom. Numerical simulations indicated that the CH2DNH2 isotopologue can be produced on icy grain surfaces within the relevant timescale (106 years); the abundance ratio, CH2DNH2/CH3NH2, can reach 10−1 under the assumption of an atomic D/H ratio of 10−2.
Hydroxyl (OH) radicals are thought to be abundantly produced on icy grains because they are an intermediate in the formation of H2O—the most abundant component of the ice mantle—and are a UV photolysis product of H2O. Therefore, the OH radical is a critical radical in chemical evolution, that is, the formation of COMs on icy grains.13) The chemical reactions involving OH radicals are activated when the temperature of the dust increases in star-forming regions. To assess the OH chemistry in chemical models, information on the surface diffusion of OH on ice prior to the reaction is desired. That is, in situ detection of the OH radicals on ice is desirable. Because of the high reactivity of OH radicals, achieving a surface density of OH on ice sufficient for detection using conventional experimental methods is difficult. Therefore, the methods typically used for analyzing solids, such as Raman spectroscopy, IR spectroscopy, and electron spin resonance spectroscopy, are inapplicable. Therefore, a new method must be established to sensitively detect OH radicals on ASW. Recently, we successfully utilized the PSD-REMPI method to detect OH radicals on ice. Details concerning the OH radical detection by PSD-REMPI101) are provided in this subsection, and its application to the surface diffusion of OH radicals on ice102) is described thereafter (Section 4.1.2).
The experimental apparatus used for OH radical detection is similar to that shown in Fig. 1a. The OH radicals on ASW are produced by photolyzing H2O using UV photons from a conventional deuterium lamp. The generated OH radicals are desorbed using a PSD laser and subsequently ionized using a REMPI laser at ∼1 mm above the surface. The (2 + 1) REMPI scheme via D 2∑− (v′ = 0) is used (see the rotationally resolved PSD-REMPI spectra in Ref. 101). “Delay time spectra”, which reflect the translational energy distribution of desorbed OH radicals, are acquired by changing the delay between the PSD and REMPI lasers, which corresponds to the TOF of OH from the surface to the focal point of the REMPI laser (Fig. 5a). The observed distribution can be fit appropriately using flux-weighted Maxwell–Boltzmann distributions at ∼3000 K (0.26 eV). This extremely high translational energy cannot be attributed to gentle desorption processes, such as the phonon-induced desorption suggested previously for the PSD of the H atom from ASW.28),30) The PSD-REMPI intensity of OH radicals is independent of the ASW thickness (Fig. 5b) and linearly dependent on the PSD laser power (Fig. 5c), indicating that the desorption is induced by a one-photon process occurring on the surface.

(a) Translational energy distribution of OH radicals photodesorbed from ASW, which was acquired by changing the delay between the PSD and REMPI laser pulses. The blue profile represents a fit of the experimental data to a single Maxwell–Boltzmann distribution at ∼3000 K. (b, c) Signal intensity of OH radicals plotted as a function of (b) ice thickness and (c) PSD laser power. These figures were derived from Ref. 101.
To gain further insight into the mechanism of the PSD process, we performed quantum chemical calculations on the OH radicals adsorbed on ASW.75),101) First, an ice cluster model for ASW comprising 159 H2O molecules was prepared. Second, the vertical excitation energy for the 2Σ+ ← 2Π transition, which corresponds to the A–X transition of isolated OH, was calculated for 18 types of binding sites on OH radicals using time-dependent density functional theory. The calculated excitation energies were generally in the range 3–4 eV (corresponding to 400–300 nm photons), which is similar to that calculated for the H2O–OH complex.103) We observed a largely red-shifted transition at 2.14 eV (580 nm) for a strongly bound OH radical on ice having a binding energy of 0.61 eV. Although this excitation energy does not exactly match the photon energy of the PSD laser (532 nm), certain OH radicals in the strongly bound sites of ASW would absorb the photons at 532 nm to be excited to an electronically excited state. These results indicate that the OH radicals detected by PSD-REMPI originate from those adsorbed on the strongly bound sites. Although the exact mechanism leading to the desorption of OH remains unclear, we proposed a mechanism based on preliminary calculations of the potential energy surface in the excited state. A conical intersection was found near the bottom of the excited state. The transition to another repulsive electronic state via this conical intersection may lead to desorption. Indeed, the measured translational energy of photodesorbed OH radicals (∼0.26 eV) was consistent with the calculated energy gap (0.24 eV) between the conical intersection and the OH + ice dissociation limit. High-level quantum chemical calculations should be performed to elucidate the exact desorption mechanism; however, calculations based on the ASW model are beyond computational feasibility. Therefore, calculations performed using OH–(H2O)n with a sufficiently large n and a sufficiently high level of theory are imperative to obtain further insight into desorption dynamics.21)
The PSD-REMPI technique for OH radicals has been adopted to study the surface diffusion of OH radicals on ice (see next subsection).102) Furthermore, the behavior of OH radicals has been clarified for scenarios with coexisting electrons on the surface, which has led to the discovery of negative current conductivity, that is, proton-hole transfer in ice. Details regarding the latter topic can be found in certain original research papers104),105) and reviews.20),21)
4.1.2. Surface diffusion of OH radicals on ice.The activation energy for the surface diffusion of OH radicals (Esd) is important for evaluating its reactions with less-mobile species, where the diffusion of OH radicals controls the reaction rate. The experimental determination of Esd is described henceforth. During the continuous production of OH radicals on ASW by UV photolysis, the PSD-REMPI signal for OH radicals on ice reaches a steady state, that is, the signal intensities do not change with time. In this scenario, the production and loss of OH radicals are balanced. The steady-state signal intensities were recorded at temperatures between 54 and 80 K and found to decrease with increasing temperature, from approximately 60 to 80 K (Fig. 6a). In other words, the loss of OH radicals accelerated at higher temperatures. Under steady-state conditions, the number density of surface OH radicals, [OH], can be expressed as
| \begin{equation} 0 = \frac{d[\text{OH}]}{dt} = f\sigma c[\text{H$_{2}$O}] - 2k_{\text{OH-OH}}[\text{OH}]^{2} - k_{\text{des}}[\text{OH}], \end{equation} | [8] |
| \begin{equation} k_{\text{OH-OH}} = D_{\text{s}} = D_{0}\exp\left(- \frac{E_{\text{sd}}}{k_{\text{B}}T} \right), \end{equation} | [9] |
| \begin{equation} -{\ln}[\text{OH}]^{2} = \ln D_{0} - \ln \frac{f\sigma c[\text{H$_{2}$O}]}{2} - \frac{E_{\text{sd}}}{k_{\text{B}}T}. \end{equation} | [10] |

(a) PSD-REMPI intensities of OH radicals photodesorbed from ASW during continuous UV irradiation under steady-state conditions at different temperatures. (b) Arrhenius-type plot between the square of OH intensities (IOH2) and reciprocal temperature, based on Eq. [10]. The red line represents a linear fit of data points at temperatures of 66, 68, 70, 72, and 74 K, which are represented by black symbols. These figures were adopted from Ref. 102.
Because the experimental determination of Esd is more difficult than that of Edes, which can be derived from TPD experiments, astrochemical models often use a simple scaling relationship Esd = fEdes, where the scaling factor f is set to be a universal value regardless of the adsorbed molecules. Consequently, a wide range of Esd values are used in models, depending on the adopted f and Edes values. Based on the determination of Esd for stable molecules,107)–109) Furuya et al. found f to be molecule-dependent.110) Additionally, through astrochemical model calculations, they confirmed the presence of the following key species whose Esd significantly affected the model predictions: O and N atoms; and HCO, CH2, and NH radicals. Therefore, the determination of Esd for these atoms and radicals is essential for obtaining better predictions using astrochemical models, and the PSD-REMPI method should play an important role in this regard.
4.2. COM formation studied using the ion-pickup method. 4.2.1. Apparatus for Cs+-ion pickup.As mentioned above, several technical requirements exist for conducting experiments on the behavior of free radicals and trace species on ice. The method should be highly sensitive to low densities, surface selective to distinguish species on the surface and in the bulk, and (ideally) nondestructive to track the time evolution of species. The PSD-REMPI method has been demonstrated to fulfill these requirements and has been successfully applied to H atoms and OH radicals on ice. One disadvantage of the PSD-REMPI method is that the target species must be gently photodesorbed by weak laser radiation; therefore, this technique cannot be feasibly employed for larger species. Moreover, the REMPI scheme cannot be applied to all species (such as methane). Consequently, we developed the “Cs+-ion pickup” method as an alternative to PSD-REMPI.
The Cs+-ion pickup method is a reactive-ion-scattering technique that is utilized in surface science and was developed by Kang et al. at Seoul National University.111) Originally, this method was extensively used to examine the physicochemical processes of molecules on clean, well-defined surfaces. However, its application to species on rough surfaces, such as ASW, has not been successful because of the roughness-induced decrease in detection efficiency. Therefore, we focused on improving the sensitivity of the Cs+-ion pickup method to permit in situ, nondestructive monitoring of the radical reactions on ASW. Because the fundamentals related to this method have been extensively described elsewhere,111) only the operating scheme of this method and the performance of our newly developed apparatus are described herein.
Schematics of the Cs+-ion pickup method are shown in Fig. 7a. Ice samples are produced on a cooled Al substrate located at the center of an ultrahigh-vacuum chamber. A low-energy Cs+ beam (kinetic energy, ∼20 eV; flux, ∼1 nA) is injected into the chamber at an angle of 45° with respect to the normal to the substrate surface. When Cs+ ions (mass number 133) scatter on the substrate, certain Cs+ ions pickup molecules (mass number M) from the outermost surface and scatter as Cs+-ion/molecule complexes (mass number 133 + M). The scattered ions are subjected to mass analysis using a QMS, whose conventional ionization source is removed. Specially designed ion lenses, which are installed in front of the QMS, significantly improve the detection sensitivity, which is up to ∼103 times greater than that of the conventional reactive-ion-scattering setup.

(a) Schematic of the Cs+-ion pickup apparatus. Cs+ ions ejected from an ion-gun scatter near the surface of an ice sample prepared on a low-temperature Al substrate. Certain Cs+ ions pick up a molecule (X) from the surface to form a Cs+–X complex and are then subjected to mass analysis using a QMS. (b) Cs+-ion-pickup mass spectrum of an ASW sample at 10 K.
Figure 7b shows the mass spectrum of an ASW sample at 10 K acquired via Cs+-ion pickup. The strongest signal at m/z 133 is attributed to the primary Cs+ ions, whereas the peak at m/z 151 (133 + 18) originated from H2O. As shown in Fig. 7b, isotopologues of water—H217O and H218O—were also detected at their natural abundances of approximately 0.04% and 0.2%, respectively. These results suggest that a detection limit of ∼10−4 monolayer coverage (1 monolayer = 1 × 1015 molecules cm−2) was achieved.
However, this method cannot separate structural isomers. As described in the next subsection, we distinguished isomers by acquiring mass spectra during the sample warm-up to identify the specific sublimation temperature of the target molecule; this method is analogous to the TPD analysis involving ionization and detection of sublimating species.
4.2.2. Formation of methyl formate on ice.Among the COMs observed in MCs and star-forming regions, the structural isomers of C2H4O2—methyl formate (MF; HCOOCH3), acetic acid (CH3COOH), and glycolaldehyde (HOCH2CHO)—are attracting considerable attention because of their known abundances; thus, understanding their formation pathways will provide insight into the chemical evolution in interstellar media. Among the aforementioned isomers, MF is most abundant in various astronomical objects.112)–114) Therefore, the formation mechanism of MF has been primarily targeted in laboratory experiments. Because solid methanol is thought to exist on the ice mantle and is thus an important precursor of the aforementioned COMs, pure solid methanol has been subjected to radiolysis and photolysis experiments.115)–117) Although MF was formed in these experiments, other products such as ethylene glycol (EG; HOCH2CH2OH) and ethanol (Eth; CH3CH2OH) were present in greater amounts. These product-yield-based relationships are inconsistent with astronomical observations, where MF is considerably more abundant than EG and Eth. The aforementioned compounds can be produced via the following reactions involving CH2OH (Eqs. [11] and [12]) and CH3O radicals (Eq. [13]):
| \begin{equation} \text{CH$_{2}$OH} + \text{CH$_{2}$OH} \to \text{HOCH$_{2}$CH$_{2}$OH (EG)}, \end{equation} | [11] |
| \begin{equation} \text{CH}_{2}\text{OH} + \text{CH}_{3} \to \text{CH}_{3}\text{CH}_{2}\text{OH (Eth)}, \end{equation} | [12] |
| \begin{equation} \text{CH$_{3}$O} + \text{HCO} \to \text{HCOOCH$_{3}$ (MF)}. \end{equation} | [13] |
The pickup mass spectrum collected during the UV irradiation of methanol adsorbed onto ASW at 10 K is shown in Fig. 8a. In this spectrum, the peaks induced by UV irradiation (indicated in red) include those of OH (17), HCO (29), and CH3O or CH2OH (31) radicals and the stable products of CO (28), H2CO (30), C2H4O2 (60), and C2H6O2 (62); the mass numbers of species are indicated in parentheses. Notably, only the CO and H2CO products were identified through the IR measurements. Unfortunately, the structural isomers, that is, masses 31, 60, and 62, cannot be distinguished in the pickup mass spectrum. Assignments were made for the stable products (masses 60 and 62) by acquiring their pickup spectra during sample warm-up. After UV photolysis, the sample temperature was gradually increased, and the mass-60 signal was continuously acquired (upper panel of Fig. 8b). The signal intensity dropped rapidly at ∼125 K due to the sublimation of the product with a mass of 60. Reference measurements were performed by depositing MF, acetic acid, and glycolaldehyde vapors on ASW; the temperature at which MF disappeared in the pickup spectra (125 K; lower panel of Fig. 8b) was remarkably consistent with that of the UV-photolysis product. Similarly, the product with a mass of 62 was assigned to methoxymethanol (MM; CH3OCH2OH) from reference measurements by depositing MM and EG vapors on Al substrate.

(a) Cs+-ion-pickup mass spectra of UV-irradiated methanol on ASW at 10 K. The peaks induced by UV irradiation are indicated in red. (b) Intensities of the mass-60 signal (m/z 133 + 60) for the UV-irradiated sample during warm-up, represented in the upper panel. The results of a reference experiment conducted using MF on ASW are shown in the lower panel. (c) Formation path diagram of MF and related COMs produced via radical–radical reactions involving CH3O radicals on icy dust surfaces. The COMs observed in a cold MC and high-mass star-forming region are indicated by an asterisk (*) and double asterisk (**), respectively. These figures were sourced from Ref. 118.
The formation of MM occurred via the reaction:
| \begin{equation} \text{CH$_{3}$O} + \text{CH$_{2}$OH} \to \text{CH$_{3}$OCH$_{2}$OH (MM)}. \end{equation} | [14] |
| \begin{equation} \text{H$_{2}$O} + h\nu \to \text{OH} + \text{H}, \end{equation} | [15] |
| \begin{equation} \text{CH$_{3}$OH} + \text{OH} \to \text{CH$_{3}$O} + \text{H$_{2}$O}. \end{equation} | [16] |
| \begin{align} &\text{CH$_{3}$OCH$_{2}$OH (MM)} + h\nu \\ &\quad \to \text{HCOOCH$_{3}$ (MF)} + \text{2H (or H$_{2}$)}. \end{align} | [17] |
Through experimental determination of the reaction pathways, a formation path diagram was constructed for MF and related COMs formed via radical–radical reactions driven by CH3O radicals on the interstellar dust surface (Fig. 8c). After the production of trace amounts of methanol on icy grains, COMs—such as MF—can be synthesized in cold MCs. In realistic icy dust grains, where CH3 and HCO radicals also exist, the formation of dimethyl ether (CH3O + CH3 → CH3OCH3) and the direct formation of MF (CH3O + HCO → HCOOCH3) may occur. The COMs originating from CH3O radicals (that is, MM, MF, and dimethyl ether) are more abundant than those originating from CH2OH radicals (EG and GA) in various astronomical objects,17),115) which is consistent with the mechanism illustrated in Fig. 8c. Finally, OH radicals were confirmed to facilitate the formation of various COMs, particularly in cold MCs.21),102)
The H2 molecule has two nuclear spin isomers: ortho and para (total nuclear spin, 1 and 0, respectively).119) According to the Pauli exclusion principle, ortho- and para-H2 molecules are coupled with the rotational states of odd and even quantum numbers, respectively. The energy difference between the lowest energy state of the isomers is 14.7 meV. In the gas phase, interconversion between the ortho and para species is forbidden, and nuclear spin conversion (NSC) occurs only via proton or H-atom exchange on a conversion timescale of 105–107 years. Because of this remarkably slow conversion, ortho- and para-H2 are often considered different species in astrochemistry. The ortho-to-para abundance ratio (OPR) of H2 is an important parameter that influences the chemistry and physics of H2 in astrophysical environments. For example, the OPR affects chemical evolution processes, such as deuterium fractionation in the gas phase.120) Moreover, because of the different heat capacities of ortho- and para-H2, the OPR affects the gas dynamics of core formation during star formation.121)
The OPR at thermal equilibrium is determined using the rotational Boltzmann distribution, and it approaches the statistical value of 3 at temperatures above ∼200 K.119) In the warm gas of molecular shock having a kinetic temperature above 300 K, the OPR is lower than the statistical value (0.5–2.0),122) indicating that the OPR is off-equilibrium with the circumstances. In other words, the OPR in astronomical objects cannot be estimated from their temperature. Moreover, the OPRs of interstellar H2 in MCs cannot be readily determined through astronomical observations because of the absence of a dipole moment in H2. Therefore, to elucidate the implications of astronomically observed OPRs, the possible mechanisms underlying the NSC of H2—particularly in MCs—should be clarified.
Compared to the gas-phase mechanisms, the NSC on dust grain surfaces is less understood. By combining the sublimation method (PSD or TPD) and detection technique (REMPI), which can selectively ionize ortho- and para-H2, we determined the NSC timescale on the surfaces of ASW and bare grain surface models (amorphous silicate and DLC) as a function of surface temperature.123)–125) The surface temperature dependence of the NSC time constants is plotted in Fig. 9. The NSC of H2 occurred rapidly on these surfaces (within ∼103 s). The mechanisms to explain the temperature dependent NSC rates were proposed in original papers.123)–125) To determine the potential effects of the NSC on dust grains on the gas-phase OPR under astrophysical conditions, we performed numerical simulations considering the rate of collision between the gaseous ortho-H2 and the grain surface, sticking probability, NSC time constant, and residence time of adsorbed H2.124),126) The simulation results indicated that the H2-related NSC processes on the surfaces helped decrease the gaseous H2 OPR in MCs.

The OPRs of polyatomic molecules such as H2O and NH3 have also been investigated for various astronomical objects to infer the chemical and thermal history of these molecules.6),23) At thermal equilibrium, the OPR of H2O reaches its statistical value (3) at temperatures above 50 K. The OPR values lower than 3 have been reported for certain star-forming regions (0.1–2.4) and proto-planetary disks (0.8).6),23),127),128) Because thermal desorption of water molecules does not typically occur in cold regions (<50 K), photodesorption is considered a plausible process that leads to the observed gaseous abundance. However, the effects of photodesorption on the OPR of H2O molecules are poorly understood. Given the lack of understanding of this process, the observed OPR value should not be linked with the formation temperature of ice; therefore, the implications of the observed OPRs of various astronomical objects remain unclear despite extensive observational studies.
Hama et al. experimentally studied the OPR of photodesorbed H2O from water ice at 10 K.129) They desorbed H2O molecules from vapor-deposited ASW or ASW produced in situ through the nonenergetic hydrogenation of solid O2 and determined the OPR (spin temperature) by REMPI spectroscopy. Interestingly, the OPR of H2O photodesorbed from 10 K ASW had a statistical value of 3, regardless of the ASW preparation method. The authors suggested that the lowest energy levels of ortho- and para-H2O molecules nearly degenerated in ice because of rotational hindrance, leading to rapid NSC between the ortho- and para-H2O (timescale: 10–100 µs) for achieving dynamic equilibrium at a statistical ratio of 3. Because the photodesorption occurs considerably faster (500 fs), the NSC during a photodesorption event is negligible. These considerations are corroborated by the determination of OPR (= 3) for the photodesorption from ice prepared from para-H2O monomers.130)
These experimental results suggest that the OPR of gaseous H2O molecules present in astronomical objects cannot be used to deduce the surface temperature of the interstellar dust where H2O formation or condensation occurs. In cold regions, the OPR of gaseous H2O molecules may decrease from 3 to lower values upon desorption via gas-phase reactions with ions such as H+ and H3O+. Therefore, to simulate the OPR values in astronomical objects, the mechanism of gaseous NSC processes should be clarified and their rate coefficients should be accurately determined.
5.3. Structure of ice mantle.The ice mantles surrounding dust grains in MCs and protoplanetary disk environments are implicitly assumed to have homogeneous layers, regardless of their composition or crystallinity. This layered structure is often considered “onion-like”. For example, Ehrenfreund et al. proposed that the icy grains toward massive protostars have spherical structures comprising a silicate core, an inner H2O-dominated polar ice mantle, and an outer apolar ice mantle dominated by CO, N2, and some O2.131) Similar layered structures—H2O-rich ice in the interior, a mixture of CO and CO2 ice in the middle layer, and CO-rich ice on the exterior—have been suggested to exist for ice in MCs by Pontoppidan et al., Boogert et al., and Öberg.7),132),133) These structures have been simply deduced based on the following sequence of ice formation processes: (i) dominant production of H2O ice (ASW) in the early stage, (ii) initiation of the reaction CO + OH → CO2 + H, and (iii) significant deposition of CO, referred to as CO freeze-out. However, the structure of the ice mantle should not be simply deduced from the sequence, but by considering the accretion rate (or formation rate) of molecules and their diffusion rate and wettability on the surface. The shape of the ice that uniformly wets the substrate or forms islands can be directly detected by transmission electron microscopy (TEM). Kouchi et al. developed an ultrahigh-vacuum low-temperature TEM apparatus134) for direct observation135) and studied the structure of astronomically relevant ice.108) They proposed a new model for the structure of icy dust grains.
The structure of the icy grains in MCs is briefly discussed herein. In conventional layered models (left image in Fig. 10), the grain comprises silicate and organic cores along with layers of ASW, amorphous CO2 (a-CO2), and amorphous CO (a-CO).136)–138) A markedly different model was suggested by Kouchi et al. (right image in Fig. 10), in which the core is covered with ASW containing nanocrystals of CO2 (CO2 I), and one CO crystal (α-CO) is attached to the ASW surface. The model established by Kouchi et al. has several astrochemical and astrophysical implications, particularly concerning chemical evolution; nonthermal desorption processes; and the collision, sticking, and sintering of dust grains. For example, in MCs at 10 K, different chemical evolutions occur simultaneously on the a-H2O and crystalline CO surfaces of a single icy grain. Successive CO hydrogenation9),10) can occur at the boundary between a-H2O and crystalline CO to produce H2CO and CH3OH. Additional information on the further thermal evolution of icy grains in MCs and protoplanetary disks can be found in the original article.108)

Conventional (left) and new (right) models for icy grains observed in low-temperature MCs.108) Each component is shown in a different color.
Laboratory experiments simulating low-temperature high-vacuum conditions deepen our understanding of the chemical evolution occurring in MCs, which produce stars and, eventually, planetary systems. Chemical processes on icy dust surfaces have long been suggested to be responsible for the large variety and abundance of molecules in MCs. Improvements in experimental methods are essential for studying these processes. For example, the development of an H-atom source that can provide the H-atom fluence expected in the typical lifetime of an MC (106 years) within a laboratory timescale can reveal the formation mechanisms of abundant organic molecules such as H2CO and CH3OH.
As discussed in Section 2.1, molecular hydrogen (H2) is the most abundant molecule in the Universe and plays an important role in several astronomical processes. Experiments have indicated the efficient production of H2 molecules on the surface of low-temperature ice dust. Low temperatures (such as ∼10 K) allow H atoms to remain on the surface for a sufficiently long time, and the surface serves as a third body to dissipate the reaction energy (4.5 eV) of the H + H → H2 reaction. Notably, this mechanism requires extremely low temperatures. Diffuse clouds, which are the precursors of MCs and are at a temperature of ∼80 K, exist in interstellar media.139) H2 molecules are abundant in diffuse clouds also, and the formation mechanism in this relatively high-temperature environment remains unclear. Although we found that the formation of H2 on a DLC surface is remarkably efficient even at 20 K, this result is valid under laboratory conditions of a high H-atom flux; in other words, the residence time of H atoms does not need to be very long.35) Therefore, the diffusive recombination reaction of physisorbed H atoms (via the Langmuir–Hinshelwood mechanism) cannot be responsible for the production of H2; however, the reaction between the H atoms colliding with the dust and those chemisorbed on the dust surface (via the Eley–Rideal mechanism) can occur even at high temperatures. Indirect evidence for H2 formation via the Eley–Rideal mechanism has been provided for an amorphous carbon surface.140),141) Direct detection of nascent H2 molecules, possibly using the PSD-REMPI method, is desirable to clarify the exact mechanism.
As described in Section 3.1, deuterium enrichment in methanol occurs efficiently via H–D substitution reactions. Among the deuterated isotopologues of methanol, those deuterated in the hydroxyl group—such as CH3OD—have not been observed in laboratory experiments (simultaneously irradiating solid CO with H and D atoms).88) In contrast, CH3OD has been detected around low-mass and high-mass protostars.142) Moreover, the CH2DOH/CH3OD ratio differs between low- and high-mass protostars (>10 and ∼1, respectively), indicating different formation pathways. The issue concerning the absence of CH3OD in the experiments has been addressed based on the experimentally revealed fact that the H–D substitution does not occur for the hydroxyl group at 10 K, which indicates that the absence is likely related to the process of formation. In the successive hydrogenation reaction of CO ($\text{CO}\overset{\text{H}}{\to}\text{HCO}\overset{\text{H}}{\to}\text{H$_{2}$CO}\overset{\text{H}}{\to}\text{CH$_{3}$O}/\text{CH$_{2}$OH}\overset{\text{H}}{\to}\text{CH$_{3}$OH}$), the first and third steps are rate-limiting and occur via quantum mechanical tunneling, whereas the other two steps are barrierless. During the simultaneous H- and D-atom irradiation of solid CO, the first and third steps occur only with H atoms because of the significant isotope effect. Because H atoms are abundant near the position where these reactions occur, the second and fourth steps may occur either with H or D atoms (according to the H/D ratio of the irradiation). The absence of CH3OD in this experiment indicates that the product of the third step is CH2OH rather than CH3O. Under the realistic conditions of icy dust surfaces, hydrogen (and deuterium) atoms are deposited at a rate of approximately one per day, indicating the lack of competition between H and D atoms in the first and third steps. Therefore, the reaction H2CO + D → CH2OD likely occurs on icy dust surfaces, although it has been shown to be less efficient than the H-abstraction reaction.89) If an extremely low H(D)-atom flux is used, the reaction rate is determined by the H(D)-atom deposition rates, but the reaction product yields are too low to be detected by IR spectrometry. Therefore, the use of highly sensitive methods such as PSD-REMPI and Cs+-ion pickup under low H-atom-flux conditions may shed light on the CH3OD formation mechanism. A new mechanism for methanol formation, CH3O + H2CO → CH3OH + HCO, has recently been suggested experimentally; however, this mechanism cannot explain the formation of CH3OD, as flagged by the authors.143)
The formation mechanisms of COMs have attracted significant attention in recent years, particularly because of the significant advances in observational studies. In particular, the Atacama Large Millimeter/submillimeter Array (ALMA) has allowed us to trace chemical evolution with ultrahigh spatial resolution. The recently launched James Webb Space Telescope (JWST) shows promise for revealing the complexity of the molecular universe. COMs are believed to be formed during the temperature increase during star formation. Light radical species, such as OH, HCO, CH3, NH, and NH2, are expected to diffuse on the surface to form new molecules. However, quantitative information on the thermally activated diffusion of these species is lacking; in various chemical models, activation energies are systematically estimated using computationally determined binding energies. The PSD-REMPI method, which was introduced in Section 4.1 for the thermal diffusion of OH radicals on ice, has proven to be a powerful technique for investigating the behavior of radicals on ice; this method will hopefully be applied to other radicals in the near future. Moreover, Cs+-ion pickup can be highly effective for elucidating COM formation mechanisms. However, as mentioned earlier, the existing apparatus exhibits poor selectivity for structural isomers. We plan to incorporate a component to distinguish structural isomers. For example, after the picking-up event, the Cs+–M complex can be accumulated in an ion trap and photodissociated to distinguish isomers.
The origin of biomolecular homochirality is a remarkably fascinating mystery related to the origin of life. Asymmetric adsorption and asymmetric synthesis on chiral inorganic crystal surfaces have been proposed as possible chiral selection processes.144),145) Recently, we found that a chiral crystalline CO (α–CO) is formed on each icy grain (Fig. 10).108),109) This can be regarded as the first chiral crystal in chemical evolution. If icy grains are irradiated with one-handed circularly polarized light during the nucleation of CO, the formed crystals may have an enantiomeric excess. Moreover, the presence of metals or high-index nanoparticles on icy dust could enhance the enantiomeric excess through plasmon-assisted asymmetric nucleation.146) A similar process may occur during the crystallization of ASW to form chiral ice crystals; moreover, amorphous methanol and ammonia crystallize in protoplanetary disks, yielding α–CH3OH and NH3 I, respectively.147) These processes have not been scrutinized experimentally and are therefore worth investigating.
The authors are grateful to colleagues who contributed to the original studies cited in this review and the technical staff at the Institute of Low Temperature Science, who helped develop the experimental apparatus. The studies discussed herein were supported in part by JSPS KAKENHI grants (JP22H00159 and JP21H01139) and a JSPS Grant-in-Aid for Specially Promoted Research (JP17H06087).
Edited by Yoshio FUKAO, M.J.A.
Correspondence should be addressed to: N. Watanabe, Institute of Low Temperature Science, Hokkaido University, Kita 19, Nishi 8, Kita-ku, Sapporo, Hokkaido 060-0819, Japan (e-mail: watanabe@lowtem.hokudai.ac.jp).
amorphous solid water
COMcomplex organic molecule
DLCdiamond-like carbon
EGethylene glycol (HOCH2CH2OH)
IRinfrared
MCmolecular cloud
MFmethyl formate (HCOOCH3); MM: methoxymethanol (CH3OCH2OH)
NSCnuclear spin conversion
OPRortho-to-para ratio
PSDphotostimulated desorption
QMSquadrupole mass spectrometer
REMPIresonance-enhanced multiphoton ionization
TEMtransmission electron microscopy
TPDtemperature-programmed desorption

Masashi Tsuge was born in 1979 in Aichi Prefecture, Japan. In 2007, he received his Ph.D. degree in Chemistry from the Tokyo Institute of Technology for spectroscopic studies on free radicals and weakly bound complexes. He subsequently joined the Niigata University of Pharmacy and Applied Life Science in 2007, where he studied molecules in strong laser fields and the ionization mechanisms of the MALDI process. From 2011 to 2013, he was a postdoc in Prof. M. Räsänen’s group at the University of Helsinki, where he participated in studies on matrix isolation spectroscopy of noble-gas-containing species and cis–trans isomerism of carboxylic acids. From 2014 to 2017, he was a postdoc in Prof. Y.-P. Lee’s group at National Chiao Tung University (Taiwan), where he employed the para-hydrogen matrix isolation technique for spectroscopic investigation of free radicals and ions. Since joining a research group at the Institute of Low Temperature Science (ILTS; Hokkaido University) in 2017, his research has focused on the reaction dynamics relevant to chemical reactions on/in interstellar grains.

Naoki Watanabe received a Ph.D. degree in atomic and molecular physics from Tokyo Metropolitan University in 1993. As a postdoctoral researcher at RIKEN until 1996, he worked on the photoionization of highly charged ions and molecules using synchrotron radiation. Since becoming a staff member at ILTS in 1996, he has investigated the physics and chemistry of solid surfaces, particularly those of solid water, at extremely low temperatures that are relevant to chemical evolution in space. He is currently a professor at Hokkaido University, and his research interests also cover gas-phase reactions, including those in low-temperature clusters.