2016 年 1 巻 論文ID: 20160003
2016 年 1 巻 論文ID: 20160003
Background: Magnetic resonance diffusion-tensor imaging (DTI) is a tool for the assessment of neural fiber integrity. We applied DTI in a patient with hearing loss that developed after bilateral putaminal hemorrhage. Case: A 59-year-old woman was referred to our outpatient clinic for sequelae diagnosis. Six years earlier, she had suffered a left putaminal hemorrhage, but almost fully recovered. Four years later, she suffered a right putaminal hemorrhage, resulting in severe left hemiparesis and hearing loss. After receiving conservative acute care treatment, she was transferred to a long-term rehabilitation facility and returned home 7 months later, when her Functional Independence Measure score was 103 points. Although the patient could not respond to auditory stimuli, her writing and reading abilities were intact. Auditory examinations indicated that the brainstem response was normal, but pure tone audiometry was at the low end of the scale (105 dB). We examined the patient’s brain using DTI, and the lesions were assessed in reference to the standard brain map transformed into her individual brain space. Fractional anisotropy and color brain maps indicated that the lesions were located within bilateral acoustic radiations. In addition, we applied fiber tracking analysis in which voxels of the medial geniculate bodies in the standard brain map were transformed into the patient’s individual brain space and then taken as seeds for the fiber tracking. The resulting image showed bilateral disruption of acoustic radiation fibers. Conclusion: By applying DTI, we identified the neuroanatomical pathology of hearing loss that developed after bilateral putaminal hemorrhage.
Recent advances in neuroimaging, including magnetic resonance imaging (MRI), have been remarkable.1) In particular, magnetic resonance diffusion-tensor imaging (DTI) enables the evaluation of neural fibers in vivo.2) This technique has been utilized in clinical settings for outcome prediction in patients after stroke, among other conditions.3) Here, we report a case of hearing loss following bilateral intracerebral hemorrhage (ICH). Assessment by DTI showed that the bilateral ICH lesions damaged the right and left acoustic radiations. To the best of our knowledge, this is the first DTI study reporting the association between hearing loss and acoustic radiation lesions in a stroke patient.
A 59-year-old, right-handed woman was referred to our outpatient clinic for sequelae diagnosis after an ICH to support her application for social welfare services. Informed consent for this case report was obtained from the patient. Six years prior to the visit to our outpatient clinic, the patient had an ICH in the left putamen. She recovered almost fully, except for slight residual numbness in the right upper extremity. Four years after the first ICH, the patient suffered a second ICH. The lesion sites of the second ICH were the putamen and thalamus in the right hemisphere (Fig. 1, left panel). The second ICH resulted in severe hemiparesis on the left side and severe hearing loss. Gross motor functions assessed on the Medical Research Council (MRC) scale4) were grade 1 for the upper and lower extremities on the left side. The patient received conservative treatments such as anti-hypertensive agents and rehabilitation during acute care and was then transferred to a long-term rehabilitation facility.5) The rehabilitation program consisted of conventional physical therapy, occupational therapy, and speech therapy for a combined daily total of up to 180 minutes every day, in accordance with Japanese guidelines for the management of stroke.6)
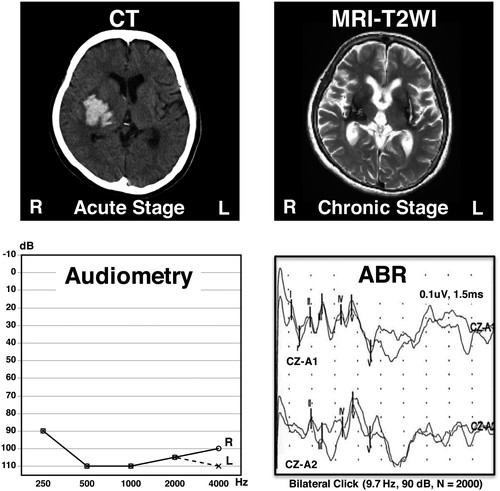
Computed tomography (CT) brain image obtained during the acute stage of the second putaminal hemorrhage (day 1, upper left panel); magnetic resonance T2-weighted image (MRI-T2WI) obtained at 20 months after the onset of the second putaminal hemorrhage (upper right panel); pure tone audiometry (lower upper left panel); and auditory brainstem response (ABR, lower right panel). L, left; R, right.
During treatment in the long-term rehabilitation facility, the patient’s hearing did not recover. Speech–language–hearing therapists examined the patient’s language ability. Although she could not respond to auditory stimuli, her language ability (Japanese) was intact: she could write Japanese (both kana and kanji) and could read written characters with vocalization and appropriate comprehension. The patient achieved a full score on the mini-mental state examination,7) indicating that her cognitive functions were intact. During rehabilitation sessions, the patient was trained to lip read to facilitate oral communication, and this gave her the ability to engage in face-to-face communication in simple sentences with family members. She was discharged home 7 months after the second ICH. Gross motor function for the left side reached MRC grade 2 for the upper extremity and grade 3 for the lower extremity, and the total score of the Functional Independence Measure8) was 103 points at discharge (73 points for the motor component and 30 points for the cognition component).
In an attempt to determine the lesions responsible for the patient’s hearing loss, an otorhinolaryngologist performed examinations including pure tone audiometry and auditory brainstem responses (ABR). The results from pure tone audiometry were 105 dB for both the right and left sides; however, ABR showed normal findings (Fig. 1). Accordingly, the patient’s hearing loss was assumed to be caused by neural damage at a level higher than the brainstem. The patient occasionally responded to loud noises (e.g., clashing or crackling sounds) in her environment, but such responses were inconsistent. The patient was then referred to our outpatient clinic. We examined her brain using DTI. The details of MRI acquisition sequences have been published elsewhere.9) For image processing, we used the FMRIB Software Library (FSL),10,11) which is a command-line programmable brain image analysis package. To better characterize the lesion sites in this patient, we enrolled three control subjects whose ages were similar to the patient’s.9) DTI data from the patient and the three control subjects were assessed in reference to a standard brain map12) and fiber tracking estimation.
Brain Mapping EstimationThe locations of the lesions were estimated in reference to the Juelich brain atlas12) defined in the standard brain space.13) The Juelich brain atlas is available in digital format with probabilistic segmentation borders of cortical and subcortical structures. For this case report, we took the segmentations for the right and left acoustic radiations.14) For the brain mapping estimation, we applied spatial transformation15) to bring the Juelich brain atlas into the patient’s individual brain space. The fractional anisotropy (FA) and color maps showed that the lesions were located in areas corresponding to the acoustic radiations in both the left and right hemispheres (Fig. 2).
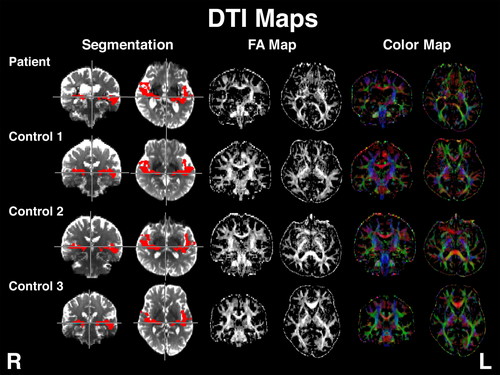
Brain images of the patient and the three age-matched control subjects. Left two columns: spatial transformation of acoustic radiations (shown in red) into the patient’s and control subjects’ individual brain spaces. Middle two columns: fractional anisotropy (FA) maps. Right two columns: color maps in which blue voxels indicate up–down, red voxels indicate right–left, and green voxels indicate back–front orientation of the neural fibers. Data for these controls were taken from our previous report.9) Control 1 is no. 27, control 2 is no. 25, and control 3 is no. 26 in the previous report.9)
To better characterize the impact of the lesions located within the acoustic radiations, we performed fiber tracking analysis.16) This technique requires “seeds” that define the starting points of the tracking. Because the neural fibers from the acoustic radiations originate in the medial geniculate bodies (MGBs),17) we sampled MGB voxels in the Juelich brain atlas. To achieve reproducibility of the analyses, we again applied spatial transformation to the MGB voxels defined in the Juelich brain atlas (refer to the Appendix for details). Figure 3 shows the results obtained from transformation of the MGB voxels for the individual subjects, indicating that the spatial transformation was successfully performed even for such a small number of voxels. Taking these voxels as “seeds,” fiber tracking analyses were successfully performed for all four subjects. The images obtained from controls 1 and 2 exhibited expansion of neural fibers (shown in red) to both sides of the primary auditory cortices in the bilateral temporal lobes. Although less evident, the image for control 3 showed that neural fibers reached the brain cortices. In contrast, the image for the patient revealed disruption of neural fibers, these neural fibers failed to reach the auditory cortices. It is worth noting that both the right and left edges of the image of the patient’s neural fibers were dull, indicating disruption of the neural fibers by the lesions.

Brain images of fiber tracking analyses. The left panel for each subject shows seed voxels, namely, medial geniculate bodies (shown in red). The right panel shows the three-dimensional image of the results obtained from fiber tracking analysis.
Cases of hearing loss following bilateral putaminal lesions have been reported previously.18,19) These articles suggest that such impairment is probably caused by damage to the acoustic radiations that pass the relatively narrow portion between the caudal end of the putamen and the internal capsule. In reference to the standard brain map transformed into the patient’s individual brain space, this case report revealed for the first time that the neural lesions of such a patient directly hit the acoustic radiations bilaterally. This case report further visualized the disruption of acoustic radiations using the fiber tracking technique.
Several previous studies have investigated the relationship between auditory dysfunction and neuroanatomical pathology using DTI.20,21,22) A case of stroke with hearing loss that developed after bilateral ischemic lesions has been reported.20) In that study, fiber tracking analysis revealed more severe fiber damage in the anterior part of the right hemisphere than in the left. The present study further employed a reproducible fiber tracking technique that could be utilized in the patient and the controls. In contrast to the brain images obtained for the control subjects, the image from the patient showed bilateral disruption of the acoustic radiations, findings that were compatible with the clinical manifestations.
Auditory dysfunction following bilateral brain lesions was sometimes termed “auditory agnosia” in previous articles.23,24) In the present case, close observation of the patient’s activities of daily living and writing ability suggested that her cognitive function was intact. Further, her social ability was good: for example, she could apply to social welfare programs. No higher brain dysfunction such as agnosia was evident. For these reasons, we simply designated her symptom as “hearing loss” in this case report.
Most of the previous DTI fiber tracking studies employed manually defined seeds, which may have resulted in less accurate reproducibility of the results. In contrast, the image analyses in this case report employed a fully computer-automated method for both the brain mapping and fiber tracking investigations. Consequently, the obtained findings were accurately reproducible. It is expected that such a consistent assessment of neural damage could be useful for revealing the lesions responsible for more complex symptoms such as aphasia, spatial neglect, and apraxia.
There are several limitations to this case report. First, the findings reported here might not be easily reevaluated because of the nature of case reports, which often deal with rare symptoms. According to the literature, cases with hearing loss are rare for supratentorial lesions.25) Second, our method for the DTI acquisition sequence (12 axes) may not have sufficient spatial resolution for detailed fiber tracking estimation.16,26) However, data from the three controls subjects enabled successful visualization of the acoustic radiations, suggesting that our image acquisition sequence was adequate for the purpose of this study. Third, despite the patient’s severe hearing disability, she inconsistently responded to loud noises such as clashing and crackling sounds. We could not assess such potentially preserved auditory acuity by using simple pure tone audiometry examination. However, such inconsistent responses to loud noises were in accordance with previous case reports.27,28) Further investigation is needed to clarify this issue.
In this case report, we used DTI to assess a case of hearing loss after bilateral putaminal hemorrhage. DTI-FA and color maps indicated that the right and left ICH lesions hit the auditory radiations bilaterally. The fiber tracking analysis employed the MGB as seed voxels and indicated disruption of the acoustic fibers. This finding suggests that the ICH lesions could be the cause of hearing loss. These observations illustrated the potential clinical applicability of DTI in the assessment of uncommon neural symptoms encountered in rehabilitation clinics.
This study was partially supported by a Grant-in-Aid for Challenging Exploratory Research, the Japan Society for the Promotion of Science (KAKENHI [15K12590]).
The authors declare that there are no conflicts of interest.
MR images were obtained using a 3.0-T MR scanner (Trio: Siemens AG, Erlangen, Germany) with a 32-channel head coil. A single-shot echo-planar imaging sequence was used for DTI acquisition: 12 images with non-collinear diffusion gradients (b=1000 s/mm2) and one non-diffusion-weighted image (b=0 s/mm2). We obtained 64 axial slices from each patient. The field of view was 230.4 mm × 230.4 mm, the acquisition matrix was 128 × 128, and the slice thickness was 3 mm without a gap. The echo time was 83 ms and the repetition time was 7000 ms.
The brain image analysis package FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/ accessed Feb. 14 2016) was used in the study. For all analytical procedures applied to the brain images, a simple programming methodology (UNIX shell script) was used on a regular Macintosh computer. FA and color maps were generated from raw DTI data (DTIFIT, a part of the FSL suite).
For spatial transformation of the brain images, a linear transformation methodology was used (FLIRT, a part of the FSL suite). DTI brain images with non-diffusion-weighted images were extracted and taken as the anatomical reference for each subject. This was transformed into the standard brain defined in the T2-weighted image (see Figs. 2 and 3). Segmentations of the Juelich brain atlas within the standard brain was then inversely transformed into the anatomical space for each individual subject.
For fiber tracking, the seed masks were defined as voxels that were within 50% probability map defined in the Juelich brain atlas transformed into individual FA images. Of these, voxels that exceeded median FA values were defined as seed masks for fiber tracking analyses (BEDPOSTX, a part of the FSL suite). To keep the analytical procedures simple, we employed default settings for all analyses.