2025 Volume 10 Article ID: 20250015
2025 Volume 10 Article ID: 20250015
Objectives: Reactive stepping is necessary to prevent falls when a person slips or trips while walking, particularly in outdoor activities. Individuals with stroke usually exhibit reactive balance impairment. Trunk training is effective for improving balance and mobility after stroke; however, its effect on reactive stepping remains unknown. This study aimed to examine the effects of trunk training on reactive stepping in community-dwelling individuals after stroke.
Methods: This study was conducted using an A-B-A single-subject design. Two community-dwelling women with chronic stroke (79 years old, 9 years post-stroke and 83 years old, 17 years post-stroke) participated in this study. The baseline (A) and intervention (B) phases lasted for 6 weeks. Specifically, the participants did not receive any intervention in phase A, whereas they performed home-based trunk training in phase B. Outcome measures included the foot-off time, maximum trunk rotation angular velocity, number of steps during forward reactive stepping following perturbation, and Trunk Impairment Scale (TIS) score.
Results: Decreased trunk rotation and step count corresponding to improved TIS score were observed in one case after the intervention. However, trunk control did not improve in the other case after the intervention, and reactive stepping kinematics remained unchanged.
Conclusions: Enhancing trunk control may improve reactive stepping in individuals with chronic stroke; nevertheless, further evidence is required.
Falls are one of the most common complications after stroke.1) Approximately 23%–73% of community-dwelling stroke survivors reportedly experienced at least one fall during a 3–12-month follow-up period.2) Falls remain a critical concern because of their potential to cause severe injuries, such as hip fractures,3,4) which typically result in individuals developing a fear of falling.4) This fear usually results in individuals restricting their physical activities,5) further deteriorating their health condition.2) Therefore, gaining a comprehensive understanding of the mechanisms underlying postural instability contributing to falls after stroke is necessary to develop interventions that improve postural balance and prevent falls.
Executing balance reactions is critical for recovering stability after balance loss. Individuals with stroke usually experience impaired reactive balance control, particularly reactive stepping dysfunction, which may increase fall risk.6) Reactive stepping in individuals with stroke is characterized by a multistep response, delayed initiation and completion of stepping, and a preference for initiating stepping with nonparetic limbs.7,8) Recent clinical trials have shown positive effects of perturbation-based balance training (PBT) in individuals after stroke. These therapies were designed to improve reactive balance control after balance loss, improve reactive balance clinical measurement scores, reduce fall rates,9) and improve reactive step kinematics, including step length, velocity, and trunk rotation.10) The findings of these studies suggested that PBT can improve stepping reactions and contribute to fall prevention in the daily lives of individuals with stroke. Given that various dynamic balance tasks, including walking and stepping over obstacles, are required during outdoor mobility, effective reactive stepping is necessary when slips or trips are induced, particularly in community-dwelling individuals with chronic stroke.
Individuals with stroke demonstrate varying levels of motor impairment, and different intensity considerations are required during training to improve balance control. Previous studies have shown that although stroke survivors demonstrated adaptation and retention of reactive stepping to slip-like perturbations, those with low paretic lower-limb functioning required training with lower perturbation intensity than high-functioning individuals.11) In addition to upper- and lower-extremity impairments, trunk motor impairment arises from stroke and follows a time course of recovery.12,13) We believe that trunk control plays a crucial role in movement control during stepping. Stroke survivors exhibit larger trunk movements than age-matched healthy adults during the stepping reaction after slip-like perturbations,14) and trunk stability during the stepping reaction constitutes a key characteristic associated with avoiding laboratory-induced falls.15) Trunk rehabilitation has been reported as an effective strategy for improving trunk control and inducing the carryover effect on sitting and standing balance and mobility after stroke.16,17) However, it remains unclear whether the carryover effect is also observed during reactive stepping after trunk rehabilitation in individuals with stroke.
We hypothesized that trunk rehabilitation would improve the reduced trunk movement during the stepping reaction and balance recovery. Therefore, this study aimed to determine whether trunk training improves reactive stepping after balance loss in community-dwelling chronic stroke survivors.
This study used an A-B-A treatment withdrawal single-subject design, which included the baseline phase (from the beginning of the study to week 6; A1) without intervention, the intervention phase (7–12 weeks; B), and the treatment withdrawal phase (13–18 weeks; A2). The intervention duration was selected based on a study finding indicating that 6 weeks of home-based trunk training increased clinical trunk control measurement in individuals with chronic stroke.18) The present study was approved by the Ethics Committee of Sapporo Medical University (approval number: 30–2-14). Prior to the study, participants were briefed on its intent and purpose, and written informed consent was obtained.
ParticipantsTwo community-dwelling participants in the chronic phase of stroke recovery were recruited for this study. The inclusion criteria were a minimum of 6 months since stroke occurrence and the ability to walk outdoors independently (Functional Ambulation Category = 5).19) Our exclusion criterion was the presence of neurological (except stroke) or musculoskeletal disorders affecting mobility.
InterventionThe intervention was a home-based trunk training program conducted at each participant’s residence.18) The trunk training components were categorized into six sets of exercises: (a) pelvic bridging, (b) sitting up, (c) trunk flexion and extension, (d) trunk lateral flexion, (e) trunk rotation, and (f) reaching (Fig. 1). Each training session constituted six exercises, each lasting 10 min, for a total of 60 min. The participants were permitted to rest if they felt fatigued during training. Overall, the intervention involved five sessions per week for 6 weeks, for a total of 30 sessions. The participants visited our laboratory every 2 weeks during the study period. During the intervention phase, the physiotherapist followed up to ensure the participants’ adherence to treatment and progressed the exercises from level 1 to level 2 if the participant showed an increased range of movement and decreased rest time during training (Fig. 2).18) The participants were instructed to fill in an exercise log, including the duration of each exercise performed daily and any comments if the participant could not exercise, to record their daily exercise compliance. A physiotherapist assessed the exercise log during the participant visits.
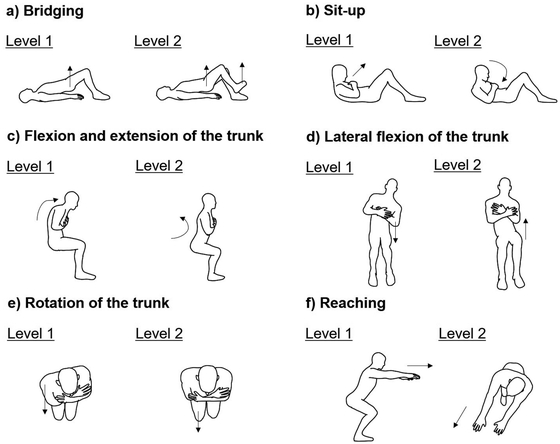
Home-based trunk training program. (a) Pelvic bridging: level 1, lift pelvis in crook lying position; level 2, lift pelvis in crook lying position and lifting the right or left leg off the floor. (b) Sitting up: level 1, lift head in crook lying position; level 2, lift head in crook lying position and rotate the upper trunk to the right or left side. (c) Trunk flexion and extension: level 1, flex and extend the upper part of the trunk without moving the lower part while sitting; level 2, anteflexion and retroflexion of the lower part of the trunk while sitting. (d) Trunk lateral flexion: level 1, touch the bed with the right or left elbow and return to the starting position while sitting; level 2, lift the right or left side of the pelvic girdle and return to the starting position while sitting. (e) Trunk rotation: level 1, move the right or left shoulder forward and backward while sitting; level 2, move the right or left knee forward and backward while sitting. (f) Reaching: level 1, forward reach a fixed point at shoulder height by forward flexing the trunk at the hips while sitting; level 2; forward diagonal reach at shoulder height while sitting.

Example images of follow-up of trunk training in the laboratory. (a) Trunk lateral flexion level 2; (b) trunk rotation level 2.
Tests and measurements, including stepping reactions, trunk control, dynamic balance measurements, and other physical and cognitive assessments, were conducted in the laboratory by two researchers. Stepping reactions were assessed every 2 weeks for the entire study period, and trunk control and dynamic balance were measured at baseline, before the intervention (week 6), after the intervention (week 12), and at follow-up (week 18). The other physical and cognitive assessments were measured at baseline and follow-up.
Stepping ReactionStepping reactions were assessed using lean-and-release experiments.20) The participant was held in a fixed position using a cable attached to a pelvic belt and asked to lean forward (Fig. 3). Subsequently, the participant was suddenly released forward, and reactive steps were induced to recover balance. This experimental technique induces steps after a random time delay with participants’ knowledge that they will lose balance by being released forward. Each participant wore a safety harness attached to an overhead bar. The participants started from a standing position with their hands resting on their chest and their feet placed on separate force plates (40 cm width, 60 cm depth, Kistler, Winterthur, Switzerland) at a standardized foot position (heel centers 0.17 m apart and 14° between the long axes of the feet).21) Next, the third and fourth force plates were placed in front of the two force plates. Shoes were worn during the test. Regarding the perturbation trials, the participants were instructed to keep their knees relaxed without hyperextension, their bodies in a straight line, and their eyes straight ahead. The duration of the random delay was in the range of 1.0–5.0 s. The pre-perturbation cable load was monitored in real time, and a load cell (LUB-B 100 KB, Kyowa Electronic Instruments, Tokyo, Japan) was placed on the lean cable to measure the target magnitude of the perturbation. The loading magnitude was 10% of body weight (BW) and corresponded to a specific body lean angle.22) In addition, the participants completed stepping reaction tasks for “usual-response conditions” and “encouraged-use conditions.” Participants were instructed to respond naturally to prevent falling to identify the preferred limb as part of the usual response. For the encouraged-use condition, participants were instructed not to step with the preferred limb but to use the opposite limb. Practice trials were initially performed for each of the usual-response and encouraged-use conditions once to minimize possible first-trial effects. After the familiarization trials, participants completed each condition three times in random order (i.e., a total of eight trials, including the familiarization trials).

Experimental setup. While wearing a safety harness that is attached to an overhead bar, the participant leans forward on a cable connected to the wall. The cable is released unexpectedly, inducing forward steps to recover balance.
Kinetic data were collected at 1000 Hz using four force plates to measure the load under each foot and a load cell to measure the load on the lean cable. An inertial sensor (TSND151; ATR-Promotions, Tokyo, Japan), equipped with triaxial gyroscopes, was attached at the participants’ seventh thoracic vertebral level to measure trunk motion, and data were collected at 100 Hz. All trials were video-recorded for offline review.
All data points were synchronized using an external computer-controlled trigger. All signals were processed offline using MATLAB R2021a (MathWorks, Natick, MA, USA). The time histories of the vertical ground reaction force (VGRF) under the legs, cable load, and trunk rotation angular velocity were low-pass filtered at a cutoff frequency of 10 Hz. We defined the lean-release time as the time at which the force recorded by the load cell was less than 1 N. The foot-off time was determined as the time from release to when the VGRF was less than 1% of BW and was recorded under the stepping limb. The maximum trunk rotation angular velocity indicated the peak value of the resultant angular velocity data (
Trunk control was measured using the Trunk Impairment Scale (TIS).23) The TIS constitutes three sub-scales: static sitting balance (3 items), dynamic sitting balance (10 items), and coordination (4 items), with maximum scores of 7, 10, and 6, respectively. The total score ranged from 0 to 23, with higher scores indicating better trunk control. A previous study has established the intra- and inter-rater reliability and concurrent validity of the TIS in individuals with stroke.23) The minimal detectable change (MDC) was 4 points.24)
Dynamic BalanceDynamic balance was assessed using the Mini-Balance Evaluation Systems Test (Mini-BESTest).25) The Mini-BESTest was developed to assess dynamic balance and comprises 14 items representing the following four balance control domains: anticipatory postural adjustments (3 items: sit to stand, rise to toes, and stand on one leg); postural responses (3 items: compensatory stepping correction in the forward, backward, and lateral directions); sensory orientation (3 items: standing on a firm surface with eyes open, standing on a foam surface with eyes closed, and standing on an inclined surface with eyes closed); and balance during gait (5 items: change in gait speed, walk with head turns, walk with pivot turns, step over obstacles, and timed up-and-go with dual task). Each item was scored on a 3-point scale (from 0 to 2), with higher scores indicating better balance, for a total score ranging from 0 to 28. The Mini-BESTest has excellent intra- and inter-rater reliability, and its concurrent validity has been established in a population with chronic stroke.26) Specifically, the MDC score for the Mini-BESTest was 3 points.26)
Other Physical and Cognitive AssessmentsLower-extremity motor function was assessed using the Fugl–Meyer Assessment (FMA), which consists of a 17-item lower-extremity sub-scale with a maximum score of 34; higher scores indicate better lower-leg function.27) This test has demonstrated good test–retest reliability.28) Lower-extremity touch and position sensation, muscle tone, range of motion, and visuospatial perception were measured using the sub-scale of the Stroke Impairment Assessment Set (SIAS). The sub-scale of the SIAS score ranges from 0 to 3 points, with a lower score indicating a more severe impairment. A previous study demonstrated that the inter-rater reliability of the SIAS items in patients with hemiparetic stroke was moderate to good.29) Cognitive ability was assessed using the Montreal Cognitive Assessment (MoCA). The total score ranges from 0 to 30 points, with higher scores indicating better cognitive abilities. Furthermore, the Japanese version of the MoCA has been reported to have excellent reliability and validity in older Japanese adults.30)
AnalysesThe paretic limb load, foot-off time, trunk rotation angular velocity, and number of steps were graphically displayed for each participant. Regarding visual analysis, the mean value and Theil–Sen slope of the measures in each phase were calculated using TrendMAD (https://manolov.shinyapps.io/Overlap/). The effect of the intervention was evaluated by assessing the change in the mean value or the trend and value of the slope in the A1 and B phases. When the effect of the intervention was observed, the mean values for the B and A2 phases were compared to evaluate the effect of its withdrawal. Changes in the TIS and Mini-BESTest total scores and sub-scores from baseline to weeks 6, 12, and 18 were also examined.
Participant 1 (P1), a 79-year-old woman, had mild lower-extremity impairment (32/34 in the lower-extremity motor scale of the FMA) and mild impairment of position sensation in the lower extremities (2/3 in the sensory sub-scale of the SIAS) (Table 1). Passive range of motion and muscle tone of the lower extremity and visuospatial perception were normal (3/3 in the sub-scale of the SIAS). P1 did not receive any support under the long-term care insurance system or rehabilitation and completed five exercise sessions per week for 6 weeks. Table 2 presents the levels of exercise in each period.
| Characteristic | Participant 1 | Participant 2 | |||
| Baseline | 18 weeks | Baseline | 18 weeks | ||
| Age, years | 79 | 83 | |||
| Sex | Female | Female | |||
| BMI, kg/m2 | 19.5 | 26.1 | |||
| Stroke type | Hemorrhagic | Ischemic | |||
| Lesion location | Right thalamus | Left medulla | |||
| Time since stroke, years | 9 | 17 | |||
| FMA score for LE (0–34) | 32 | 32 | 33 | 33 | |
| Touch sensation for LE (0–3) | 3 | 3 | 2 | 2 | |
| Position sensation for LE (0–3) | 2 | 2 | 3 | 3 | |
| Muscle tone for LE (0–3) | 3 | 3 | 3 | 3 | |
| Range of motion for LE (0–3) | 3 | 3 | 3 | 3 | |
| Visuospatial perception (0–3) | 3 | 3 | 3 | 3 | |
| MoCA score (0–30) | 25 | 25 | 26 | 26 | |
| Fall in past year, yes/no | No | Yes | |||
BMI, body mass index; LE, lower extremities.
| Exercise | Participant 1 | Participant 2 | |||||
| 6–8 weeks | 8–10 weeks | 10–12 weeks | 6–8 weeks | 8–10 weeks | 10–12 weeks | ||
| Bridging | 1 | 2 | 2 | 1 | 2 | 2 | |
| Sit-up | 1 | 1 | 2 | 1 | 2 | 2 | |
| Flexion and extension | 1 | 1 | 2 | 2 | 2 | 2 | |
| Lateral flexion | 1 | 1 | 1 | 1 | 1 | 2 | |
| Rotation | 1 | 1 | 2 | 1 | 1 | 2 | |
| Reaching | 1 | 2 | 2 | 2 | 2 | 2 | |
P1 showed improvements in the TIS total score from baseline and before the intervention (11 points each) to after the intervention (19 points) (Table 3). Dynamic sitting balance and coordination of the TIS sub-score improved from baseline and before the intervention to after the intervention. The effect of the intervention was maintained at follow-up. Although P1 exhibited slight changes in the Mini-BESTest total score (Table 2), the change was within the MDC. Notably, the score for the reactive sub-scale of the Mini-BESTest recovered from 2 at baseline and before the intervention to 3 after the intervention and at follow-up. P1 showed no change in FMA, SIAS, or MoCA scores after the intervention.
| Measure | Sub-score | Participant 1 | Participant 2 | |||||||
| Baseline | 6 weeks | 12 weeks | 18 weeks | Baseline | 6 weeks | 12 weeks | 18 weeks | |||
| TIS | Total (/23) | 11 | 11 | 19 | 19 | 18 | 17 | 20 | 20 | |
| Static (/7) | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| Dynamic (/10) | 2 | 2 | 7 | 7 | 9 | 8 | 9 | 9 | ||
| Coordination (/6) | 2 | 2 | 5 | 5 | 2 | 2 | 4 | 4 | ||
| Mini-BESTest | Total (/28) | 17 | 16 | 18 | 18 | 19 | 18 | 20 | 20 | |
| Anticipatory (/6) | 4 | 4 | 5 | 4 | 3 | 3 | 4 | 3 | ||
| Reactive (/6) | 2 | 2 | 3 | 3 | 4 | 3 | 4 | 4 | ||
| Sensory (/6) | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | ||
| Gait (/10) | 8 | 7 | 7 | 8 | 8 | 8 | 8 | 9 | ||
Figure 4 shows kinetic variables for stepping in each phase. Decreasing trends for the foot-off time (slope = −45.7, Fig. 4b), maximum trunk rotation angular velocity (slope = −11.6, Fig. 4c), and number of steps (slope = −0.2, Fig. 4d) were observed in the A1 phase, whereas the paretic limb load (Fig. 4a) showed an near-stable trend during this period. The trend for the foot-off time (slope = −6.5) was near stable in the B phase. In contrast, the maximum trunk rotation angular velocity (slope = −10.0) and number of steps (slope = −0.2) still showed decreasing trends. Furthermore, the mean value decreased from the A1 phase (trunk rotation angular velocity, mean = 108.9; number of steps, mean = 1.9) to the B phase (trunk rotation angular velocity, mean = 92.0; number of steps, mean = 1.4). The lower values of maximum trunk rotation angular velocity and number of steps in the A2 phase (trunk rotation angular velocity, mean=83.0; number of steps, mean=1.0) indicated that the effect of intervention remained in the A2 phase. Table 4 summarizes the number of trials requiring assistance under the usual-response condition and the inability to step with the non-preferred limb under the encouraged-use condition. P1 required no assistance during any trial. The frequency of the inability to step with the non-preferred limb under the encouraged-use condition decreased during this study and was rarely observed after the intervention.

Kinetic variables for reactive stepping during each phase. (a) Stepping limb load, (b) foot-off time, (c) peak trunk rotation angular velocity, and (d) number of steps in forward reactive stepping from baseline (Base) to the end of the study period. Dotted lines, level of the mean; solid lines, slope; BW, body weight; wk, weeks.
| Baseline | 2 weeks | 4 weeks | 6 weeks | 8 weeks | 10 weeks | 12 weeks | 14 weeks | 16 weeks | 18 weeks | |
| Assists (n) | ||||||||||
| Participant 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Participant 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Inability to step with non-preferred limb (n) | ||||||||||
| Participant 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 0 |
| Participant 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Participant 2 (P2), an 83-year-old woman, had mild lower-extremity impairment (33/34 in the lower-extremity motor scale of the FMA) and mild impairment of touch sensation in the lower extremities (2/3 in the sensory sub-scale of the SIAS) (Table 1). Passive range of motion and muscle tone of the lower extremity and visuospatial perception were normal (3/3 in the sub-scale of the SIAS). P2 similarly did not receive any support under the long-term care insurance system or rehabilitation and completed five exercise sessions per week for 6 weeks.
The TIS total score showed slight change from baseline (18 points) and before the intervention (17 points) to after the intervention (20 points) (Table 3). In contrast, the Mini-BESTest total score did not change during the follow-up period (Table 3). P2 also showed no change in FMA, SIAS, or MoCA scores after intervention.
Similar to P1, P2 showed decreasing trends for the foot-off time (slope = −12.8), maximum trunk rotation angular velocity (slope = −6.9), and number of steps (slope = −0.3) in the A1 phase. However, the maximum trunk rotation angular velocity (slope = 1.1) and number of steps (slope = 0.0) remained relatively stable in the B phase. The mean values in the B phase (trunk rotation angular velocity, mean = 96.4; number of steps, mean = 2.2) were only slightly different from those in the A1 phase (trunk rotation angular velocity, mean = 103.9; number of steps, mean = 2.5). P2 did not require assistance during any trial and could step with the non-preferred limb under the encouraged-use condition, except for one trial at baseline (Table 4).
The findings of this study indicate that home-based trunk training in individuals with chronic stroke and impaired trunk motor function can modify reactive stepping. The intervention was performed by the participants who had mild motor impairment of the lower extremities and could independently ambulate. Variations in trunk control were observed in both participants at baseline; however, the positive effect of the intervention was observed only in the participant with lower trunk functioning and not in the other with higher functioning. Both participants demonstrated adaptive changes from repeated perturbations in reactive stepping before the intervention; however, only the participant with improved trunk control demonstrated improvements in reactive stepping resulting from the intervention for trunk control.
P1 experienced a positive effect of home-based trunk training that improved trunk control, and the TIS score change was greater than the MDC, whereas P2 did not experience such changes. Both participants reported high compliance with exercise and completed five sessions per week for 6 weeks. The intensity and duration of training performed by P1 were sufficient to improve trunk motor control, particularly dynamic sitting balance and coordination, including adjusting to weight shifts and performing controlled movements during functional tasks. The results of this study suggest that home-based trunk training can improve trunk control, and these effects transfer to reactive balance, which contributes to fall prevention even for individuals with chronic stroke who engage in outdoor walking. However, the degree of improvement in the TIS score did not surpass the MDC for P2. Moreover, P2 demonstrated a higher TIS score at baseline than the participants of a previous study following trunk training (13.8±1.3 points in the TIS).18) Therefore, a more challenging task may be required to improve trunk control. For example, task-specific trunk exercises performed on an unstable surface (physio ball) more effectively improved trunk control than those performed on a stable surface by individuals with acute stroke.31) Physiotherapists should appropriately adjust assignment difficulty when guiding individuals with chronic stroke through home-based trunk training. In particular, dynamic treatment instruments may be beneficial for improving trunk control in individuals with higher trunk function.
We observed that repeated perturbation modified the mean and slope values of the foot-off time, trunk rotation angular velocity, and number of steps in both participants with stroke during the A1 phase. A previous study reported rapid adaptation in reactive stepping during 11 trials of slip-like stance perturbation and retention 3 weeks after training in individuals with chronic stroke.11) In the present study, the number of forward-induced steps was six (including both the usual-response and encouraged-use conditions) for each session and was assessed every 2 weeks, which could be sufficient to change reactive stepping and retention after the session. The stepping limb load before cable release did not change during the follow-up period. Specifically, the asymmetric stance before cable release influences the stepping kinematics after perturbation,22) and our results indicated that the participants did not adjust their posture before cable release. Previous studies have characterized adaptation from repeated perturbations in stroke survivors with greater step length10,11) and decreased trunk rotation, which reduces the number of steps or frequency of falls,10) increases the stepping threshold,32) and improves postural stability.10,15) Decreased trunk rotation was observed in this study, which could have contributed to the decreased number of steps. Our participants showed improved foot-off time during the A1 phase. Given that a delayed time to initiate a step is associated with increased fall rates during inpatient stroke rehabilitation,33) a shortened foot-off time may contribute to improving dynamic stability and preventing falls in individuals with stroke.
Importantly, in the current study, the carryover effect of improving trunk control after the intervention on reactive stepping was associated with a decreased trunk rotation angular velocity and number of steps in one participant with stroke during the B phase, and the effect was sustained even after the intervention was completed. Conversely, the other participant showed less change in trunk control after the intervention, and their reactive stepping kinematics remained unchanged. Trunk training after stroke leads to biomechanical improvements during walking based on factors such as walking speed, step length, trunk kinematics, and center-of-mass excursions.17) Consistent with the findings of a previous study, our result emphasized the effect of home-based trunk training on improving reactive stepping. An upright trunk position must be maintained during the stepping reaction to attain a single-stepping strategy after loss of forward balance.34) Improved trunk control leads to decreased trunk movements during forward reactive stepping, indicating an arrest-induced whole-body rotation, which could reduce the number of steps. In addition, the ability to initiate a step with a non-preferred limb may improve after trunk training. The capacity of both limbs to respond to instability correlates with the risk of falls after hospital discharge in individuals with stroke.6)
This study had some limitations. Considering the study design, generalizing the effects of the intervention on individuals with chronic stroke who have mild sensory and motor impairments is challenging. Given that the intervention was a home-based program, potential motivational differences or environmental influences during the unsupervised home training might have contributed to variability in training outcomes. The experimental protocol may have affected the results of the study because the participants were initially inclined forward to induce steps after cable release. Although forward falls were unpredictable, the direction to fall and the required steps to recover balance were known. The short time duration from forward-leaning to cable release during the lean-and-release experiment may have increased predictability and pre-planning responses. Therefore, the observed reactive stepping performance may not be the same as real-life behavior aimed at preventing falls. Although clinical scores, such as lower-extremity sensorimotor function and trunk control, were similar between P1 and P2 after the intervention and at follow-up, the stepping reaction kinematics were largely different. Consequently, the effects of confounding factors, such as age34,35) and fear of falling,36) should be considered. We only investigated the stepping responses to forward-fall postural perturbations. Although individuals with stroke may exhibit difficulty in executing stepping reactions to lateral and backward perturbations,37) we did not investigate this carryover effect in different directions. Therefore, further research is required to clarify whether trunk training could accelerate the improvement of reactive stepping and whether the effect would transfer to reactive stepping in different directions.
Although the findings of this study are preliminary because of the small sample size and exploratory design, they showed the effects of home-based trunk training on reactive stepping following forward balance loss in individuals with chronic stroke. Enhancing trunk control improved reactive stepping, as evidenced by the decreased trunk rotation during stepping and the number of steps. The implications of these findings were supported by the other participant, who did not experience changes in trunk control after trunk training and showed minimal alterations in reactive stepping. However, the generalizability of the carryover effect of trunk training on reactive stepping in individuals with stroke remains limited, warranting further investigation.
This study was supported by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (Grant 20K19415).
The authors declare no conflict of interest.