2022 年 7 巻 論文ID: 20220033
2022 年 7 巻 論文ID: 20220033
Objectives: This study examined the immediate effects of neuromuscular electrical stimulation (NMES) on the dynamics of oropharyngeal structure and laryngeal vestibular closure (LVC) in healthy subjects.
Methods: Ten healthy male volunteers participated in this controlled, before-and-after, videofluoroscopic swallowing pilot study. The study was conducted in four phases (each performed twice): (1) saliva swallow (SS) before evaluation (BEFORE), (2) NMES while at rest with no SS (NMES AT REST), (3) SS during NMES (DURING NMES), and (4) SS to examine the aftereffects of NMES (AFTER). We measured distances that oropharyngeal structures moved in the NMES AT REST phase, and we analyzed the kinematics of saliva swallowing primarily in the BEFORE and AFTER phases.
Results: Four changes in the morphology of the oropharyngeal structure caused by NMES AT REST were statistically significant: anterior–upward displacement of the hyoid bone and larynx, stretch of the laryngeal vestibule, and posterior ridge of the tongue root. Regarding the kinematics measured during SS, although there was no significant change in LVC reaction times, LVC duration in the AFTER phase was significantly longer than BEFORE. Regarding maximal displacement of the hyoid bone, there was significantly greater movement AFTER than BEFORE. As additional exploratory outcomes, the velocity of hyoid bone movement was significantly slower, and the hyoid-to-larynx approximation was significantly smaller, DURING NMES than AFTER.
Conclusions: Longer duration of LVC might be caused by adaptive learning with NMES-induced structural changes in the oropharynx. Further clinical studies are warranted to determine whether this approach improves dysphagia, which impairs LVC.
Laryngeal vestibule closure (LVC) is the primary mechanism preventing penetration or aspiration during swallowing. Given that impaired LVC causes unsafe swallowing, rehabilitation therapy for LVC dysfunction is necessary.1) Previous studies have shown that effortful swallowing,2) chin-down posture,3,4) and neuromuscular electrical stimulation (NMES)5,6) can shorten LVC reaction time (LVCrt) and extend laryngeal vestibule closure duration (LVCd). Among these, NMES is the most frequently used method for treating dysphagia in the USA.7)
It has been suggested that NMES promotes hyoid bone and larynx anterior–superior movement8) and velocity,9) and rapid movement of the hyoid bone contributes to improved LVC.10) However, various parameters have been proposed for NMES, including device selection, stimulation site, and device settings. Of these, Ampcare’s effective swallowing protocol—Ampcare ESP™—has the following features: 1) the electrodes are specially designed to match the curvature of the jaw line, and electrical stimulation occurs evenly across the surface of the electrode; 2) a “Restorative Posture Device” holds the electrodes fixed in place, improves conduction, and assists with proper cervical alignment; and 3) the training combines effortful swallowing during NMES with classical exercises that facilitate contraction of the suprahyoid muscle group.11) A recent study reported that Ampcare ESP™ has an immediate effect of shortening LVCrt.5) However, it has also been reported that the simple act of swallowing saliva (without effort) during NMES decreases the forward movement of the hyoid bone.12) In addition, there are some doubts about the effectiveness of NMES on LVC (depending on the settings),6) and the mechanism by which NMES affects LVC is yet to be elucidated. It is therefore difficult to determine the most appropriate treatment settings. We considered whether “simplified” Ampcare ESP™ (i.e., combining effortful swallowing with NMES, which intervenes with electric current above the motor threshold level) would affect LVC without using resistive exercises (which facilitate contraction of the suprahyoid muscle group). We also considered how this protocol affects the dynamics of the oropharyngeal structure including the hyoid bone. As an initial step, we conducted a controlled before-and-after pilot trial to assess the immediate changes in LVC and oropharyngeal structure with NMES in healthy volunteers. The preliminary result of this study has been presented elsewhere.13)
Ten healthy male volunteers (mean age 30.7 years, SD 2.9 years) participated in this study. Participants were included in the study if they had no contraindications to NMES, such as a history of neurological disorder, laryngopharyngeal disorder, upper gastrointestinal surgery, or deglutition disorder caused by other factors. In addition, the participants had no problems with skin on the neck and no serious heart disease. Thorough written and verbal explanations of the study were provided to all participants, and written consent was obtained from each (Approval No. 160008, 28-27, Ethics Committee of Kagoshima University). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Equipment and Stimulation ConditionAn Ampcare ES™ unit (Ampcare, Fort Worth, TX, USA) was used for NMES (Fig. 1). The stimulation pulse was a symmetric biphasic square waveform, with a pulse width of 50 μs and frequency of 30 Hz. A pulse rate of 30 Hz was chosen to produce muscle contraction by small muscle groups with the intention of stimulating contraction without fatiguing them.5) The intensity of the electrical current was adjusted to produce the maximal evoked contraction that allowed voluntary swallowing exercises without discomfort. NMES was delivered to the targeted musculature with a stimulation time of 5 s during the voluntary swallowing exercise and a resting interval of 25 s. The Ampcare ESP™ therapy system is FDA-cleared for muscle re-education by application of external stimulation to the muscles necessary for pharyngeal contraction.11)

Attachment sites for surface electrodes. Left panel: E-series electrode (Ampcare, Fort Worth, TX, USA). Right panel: electrical stimulator, Ampcare ES™ unit, and Restorative Posture Device (Ampcare). Red dots, mylohyoid; red crosses, anterior belly of the digastric geniohyoid.
All participants were clean-shaven on the submental region in advance to increase electrode adhesion, and the skin in the submental region was cleaned with alcohol before electrodes were attached. The surface electrodes (E-series, Ampcare) were located to ensure that electrical stimulation occurred evenly across the surface of the electrode, resulting in comfortable treatment (Fig. 1, left panel). The electrodes were arranged over skin areas that are anatomically defined as the motor points of the geniohyoid, the mylohyoid, and the anterior belly of the digastric muscles. This allowed NMES to maximize the evoked tension, while minimizing the dose of the injected current and the level of discomfort.14) At the time the electrodes were affixed, care was taken to avoid touching the mandible so as not to cause pain to the participants. The participants were seated in a wheelchair in an upright position and wore a Restorative Posture Device (Ampcare) (Fig. 1, right panel) to facilitate proper cervical alignment. Blood pressure, pulse rate, and oxygen saturation concentration were measured before and after NMES. To confirm safety, participants answered a questionnaire regarding pain and mood disorder after completion of the study.
Videofluoroscopic Swallowing StudyVideofluoroscopic swallowing study (VFSS) was projected in a sagittal section and a 2-cm-diameter wire loop was placed on the middle mandible as a reference of the radiographic length. The evaluation was conducted in four phases: (1) saliva swallow (SS) twice before evaluation (BEFORE), (2) NMES twice while at rest with no SS (NMES AT REST), (3) SS twice during NMES (DURING NMES), and (4) SS twice to examine the aftereffects of NMES (AFTER). The duplicate measures at each phase were averaged (Fig. 2). The evaluator instructed the subjects to swallow as strongly as possible for the SS task in the BEFORE, DURING NMES, and AFTER phases. To test the aftereffect, the initial perturbation effect, and the trial effect, we compared the averaged values of measurements (described later) at each phase as follows: primary outcome, 1) BEFORE versus AFTER; exploratory outcomes, 2) BEFORE versus DURING NMES and 3) AFTER versus DURING NMES.
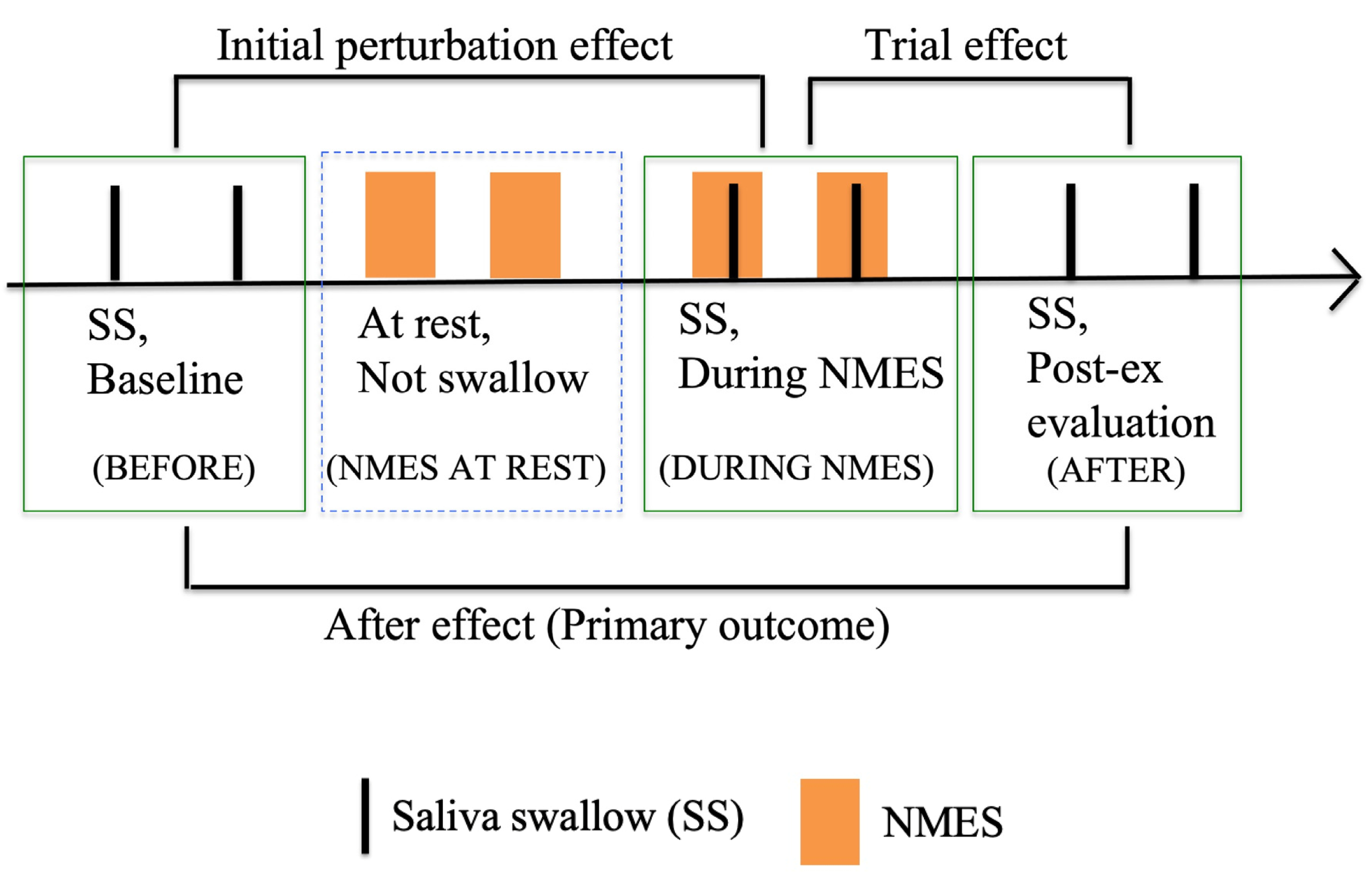
Four phases to analyze kinematics in the videofluoroscopy. BEFORE, twice saliva swallow (SS) as baseline; NMES AT REST, twice neuromuscular electrical stimulation (NMES) at rest (between SS); DURING NMES, twice SS during NMES; AFTER, twice SS to examine aftereffects of NMES. Two measurements were averaged from each phase.
The evaluator replayed each VFSS frame-by-frame (originally recorded at 30 frames per second), measured the distances moved by the oropharyngeal structures in the NMES AT REST, and analyzed the kinematics of saliva swallowing in the BEFORE, DURING NMES, and AFTER phases. We used Swallowtail ver.1 (Belldev Medical, Arlington Heights, IL, USA) to perform all measurements. Coordinates were calculated relative to the anterior–inferior margin of the line connecting the second and fourth cervical vertebrae. Data among each task were averaged and are expressed as mean and standard deviation (SD) or median and interquartile range.
Distances Moved by Oropharyngeal Structures During the NMES AT RESTDisplacement of each oropharyngeal structure (hyoid bone, larynx, laryngeal vestibule, and tongue root) was defined as the distance from the resting position without NMES (value 0, zero position, as the reference) to the highest (or lowest) position during “NMES at rest.” In this study, we regarded this displacement as “the effect of NMES on oropharyngeal structures at rest.” For assessing hyoid bone and larynx movements, measurement points were placed on the most anterior–inferior aspect of the hyoid bone (‘a’ in Fig. 3)15) and the most anterior–superior aspect of the subglottal air column16) (‘b’ in Fig. 3), respectively. The displacement of the laryngeal vestibule was defined as the distance between the upper end of the arytenoid cartilage and the lower dorsal aspect of the epiglottis base (‘c’ in Fig. 3). The posterior movement distance of the tongue root was measured as the minimum distance of the line connecting the tongue root to the pharyngeal wall perpendicular to the reference axis (‘d’ in Fig. 3).

X-Ray photograph combining two phases: “at rest” and highest position during “NMES AT REST”. Two videofluoroscopy images, “at rest” (i.e. without NMES) and peak of “NMES AT REST”, are composited to measure distances moved by oropharyngeal structures during the NMES AT REST. Oropharyngeal structures “at rest” (green points or green solid lines): a, hyoid bone (the most anterior–inferior aspect of the hyoid bone); b, larynx (the most anterior–superior aspect of the subglottal air column); c, distance of laryngeal vestibule (between the upper end of the arytenoid cartilage and the lower dorsal aspect of the epiglottis base); d, minimum distance between the tongue root and the pharyngeal wall (to measure dorsal movement distance of the tongue root). Oropharyngeal structures at the highest position during “NMES AT REST” (red points or red dotted lines): a’, b’, c’, and d’ correspond, respectively, to the same structures as those for “at rest”. The reference axis is the line connecting the lower ends of the cervical vertebrae (C2–C4).
Kinematics during SS in the BEFORE, DURING NMES, and AFTER phases were assessed in terms of the maximal displacement of the hyoid bone and larynx, velocities of the hyoid bone and larynx movements, hyoid to larynx approximation, LVCrt, and LVCd. Definitions of the measured items are as follows.
For the maximum distance of hyoid bone and larynx movement, the distance moved between the following two points was measured. Measurement was initiated one frame before onset of the hyoid and larynx movement burst, and was terminated at the point of maximal displacements of the hyoid and the larynx. The velocities of hyoid bone and larynx movements were calculated as [maximal displacement (cm)/time (s)]. Approximate distance between hyoid bone and larynx (hyoid to larynx approximation, HLx) was analyzed one frame before the onset of hyoid movement for swallowing without hesitation.17) Measurement of LVCrt was initiated at the time of onset of sustained superior movement of the arytenoids toward the laryngeal surface of the epiglottis and terminated when the arytenoids contacted the epiglottis and the supraglottal air space within the vestibule was completely sealed. Measurement of LVCd was initiated at the endpoint of the LVCrt measurement and terminated at the onset of arytenoid descent from the epiglottis (onset of open), which was verified by reappearance of the supraglottal air space (Table 1). As the primary outcome, the immediate aftereffect was assessed by comparing the BEFORE and AFTER measurements. As exploratory outcomes, the initial perturbation effect was assessed by comparing the BEFORE and DURING NMES measurements, and the trial effect was assessed by comparing the DURING NMES and AFTER measurements (Fig. 2).
| Event | Definition | |
| Maximal displacement of hyoid bone/larynx (cm) | Onset | One frame before onset of the hyoid and larynx movement burst |
| Termination | The maximal displacements of the hyoid and the larynx | |
| Maximal velocities of hyoid bone/larynx (cm/s) | The maximal velocities of hyoid bone/larynx are: maximal displacements/time | |
| HLx (cm) | Distance between hyoid bone to larynx approximation was analyzed one frame before onset of consistent hyoid rise for swallowing | |
| LVCrt (ms) | Onset | Onset of sustained superior movement of the arytenoids toward the laryngeal surface of the epiglottis |
| Termination | Arytenoids contacted the epiglottis, and the supraglottal air space within the vestibule was completely sealed | |
| LVCd (ms) | Onset | Endpoint of the LVCrt measure |
| Termination | Onset of arytenoid descent from the epiglottis, verified by reappearance of the supraglottal air space | |
Normality was evaluated by using the Shapiro–Wilk test. To analyze “the effect of NMES on oropharyngeal structures at rest,” displacement of each oropharyngeal structure at each of the four points measured with NMES and without NMES (value zero as the reference) was tested with a paired t-test. Differences among the BEFORE, DURING NMES, and AFTER periods were used to compare effects of NMES on saliva swallowing; choice of test depended on the nature of the data. Data that followed a normal distribution were analyzed with a t-test and data that did not were analyzed with Wilcoxon’s signed-rank test. All analyses were performed with SPSS version 26 (IBM Corporation, Armonk, NY, USA). P values less than 0.05 were considered statistically significant, and the effect size (r) was calculated as r=√(t2/(t2+df)).
NMES was applied safely: it produced neither adverse effects nor complications such as hypotension, arrhythmia, or syncope resulting from carotid body stimulation and laryngeal spasm. None of the participants experienced discomfort before, during, or after the intervention. The participants had an average motor stimulation intensity of 10.4 (SD 1.4), which is roughly equivalent to 50 mA.
Effect of NMES on Oropharyngeal Structures at RestThe average change in anterior–superior displacements of the hyoid bone and larynx were 0.40 cm (95% confidence interval [CI] 0.32–0.49 cm; P<0.01, r=0.9) and 0.28 cm (95% CI 0.22–0.34 cm; P<0.01, r=0.9), respectively. The average change in distance for the laryngeal vestibule was 0.27 cm (95% CI 0.21–0.33 cm; P<0.01, r=0.9), and the average change in dorsal movement of the tongue root was 0.21 cm (95% CI 0.11–0.31 cm; P<0.01, r=0.7).
Effects of NMES on Kinematics During Saliva SwallowIn examining the aftereffect, the average maximum movement of the hyoid bone in the AFTER period was significantly greater than BEFORE: 1.3 cm (SD 0.3 cm) versus 1.1 cm (SD 0.4 cm) (P=0.01, r=0.58). The average change in the LVCd in the AFTER period was significantly greater than BEFORE: 326 ms (SD 96 ms) versus 257 ms (SD 110 ms) (P=0.04, r=0.45).
In examining the initial perturbation effect (BEFORE vs DURING NMES), no significant difference was found in any of the measures. In examining the trial effect (AFTER vs DURING NMES), the average change in the velocity of the hyoid bone in the AFTER period was significantly greater than DURING NMES: 2.8 cm/s (SD 0.7 cm/s) versus 2.2 cm/s (SD 0.8 cm/s) (P<0.01, r=0.7). The average distance in the HLx was significantly greater AFTER than DURING NMES: 3.6 cm (SD 0.4 cm) versus 3.5 cm (SD 0.3 cm) (P=0.03, r=0.46) (Table 2).
| Hyoid bone | Larynx | HLx (cm) | LVCrt (ms) | LVCd (ms) | |||
| Displacement (cm) | Velocity (cm/s) | Displacement (cm) | Velocity (cm/s) | ||||
| BEFORE | 1.1 (0.4) | 2.6 (1.0) | 1.7 (1.5−2.1) | 3.5 (1.3) | 3.6 (0.5) | 454 (110) | 257 (110) |
| DURING NMES | 1.2 (0.3) | 2.2 (0.8) | 1.9 (1.6−2.1) | 3.5 (1.1) | 3.5 (0.3) | 509 (154) | 331 (139) |
| AFTER | 1.3 (0.3)** | 2.8 (0.7)††† | 1.9 (1.6−2.1) | 4.0 (0.9) | 3.6 (0.4)† | 431 (108) | 326 (96)* |
*P<0.05, **P=0.01 relative to BEFORE (primary outcome). †P<0.05, †††P<0.01 relative to DURING NMES (exploratory outcome).
Data among each task are averaged and shown as mean (SD) or median (first quartile–third quartile) representing effects of NMES on saliva swallowing BEFORE, DURING NMES, and AFTER. As the primary outcome, we compared the BEFORE and AFTER measurements. As exploratory outcomes, we compared the BEFORE and DURING NMES measurements and the DURING NMES and AFTER measurements. Data that followed a normal distribution were subjected to a t-test; data that did not were subjected to Wilcoxon’s signed-rank test. The asterisks show significant differences compared with BEFORE. The daggers show significant differences compared with DURING NMES.
This study used healthy subjects to investigate the immediate effects of NMES on the suprahyoid muscles, which play an important role in inducing LVC, by using an Ampcare ESP™ kit. For the purpose of facilitating the suprahyoid muscles, electrodes were placed on the motor points of the left and right geniohyoid muscles, digastric muscles, and mylohyoid muscles; in addition, we adjusted the maximum intensity current to allow voluntary swallowing without discomfort.
As a result, changes in dynamics and oropharyngeal structure demonstrated anterior–superior movement of the hyoid bone and larynx during NMES AT REST. Safi et al. recently reported the immediate effects of spatiotemporal measurement of the dynamics of oropharyngeal structures in NMES at rest using fluoroscopy.12) NMES AT REST in our result was generally in agreement with their results; that is, the anterior–superior movement that occurred in the hyoid and larynx.12) However, the distance of hyoid and larynx movement was greater in our study.12) Barikroo et al. reported that a shorter pulse duration penetrated more deeply into the muscles involved in swallowing18) and moved the hyoid bone forward.19) In the setting of NMES in our study and that of Safi et al., the same frequency (30 Hz) was used and the stimulation intensity was similar (mean 50 mA vs. mean 55.7 mA),12) but the present pulse duration (50 µs) was shorter than that of Safi et al. (250 µs).12) Therefore, the setting we chose might be more efficient in moving the hyoid bone forward.
In addition, a significant increase in the displacement of the laryngeal vestibule implies stretching of the laryngeal vestibule, and a significant increase of posterior movement of the tongue root implies a posterior ridge (protrusion) of the tongue root. Previous studies of NMES have not analyzed oropharyngeal structures other than the hyoid bone and larynx.5,6) Therefore, this is the first report of spatiotemporal measurement of the dynamics of these structures before and after NMES using fluoroscopy.
The velocity of hyoid bone movement was significantly slower DURING NMES than AFTER, and the aftereffect showed a significant increase in the distance moved. Shimizu et al. found that transcutaneous electrical stimulation of the entire suprahyoid muscle group may limit anterior–superior movement of the hyoid bone because the stimulation not only affects muscles for anterior–superior movement of the hyoid bone but also simultaneously affects muscles for posterior movement (i.e., the action of the stylohyoid muscle or the posterior belly of the digastric muscle).20) Therefore, it is possible that NMES contracted not only the geniohyoid, digastric, and mylohyoid, but also the stylohyoid and the posterior belly of the digastric and acted to resist anterior–superior movement of the hyoid bone during swallowing. Safi et al. performed an empty swallow (without effort) during NMES and reported decreased anterior movement of the hyoid bone.12) In the current study, effortful swallowing was performed BEFORE, DURING NMES, and AFTER, and the anterior–superior movement of the hyoid bone DURING NMES was not significantly different from BEFORE. Extension of the distance moved by the hyoid bone in the aftereffect was considered to be the result of many motor units being mobilized for the anterior–superior movement of the hyoid bone,21) and the effect was sustained even after the end of NMES.
HLx in the AFTER phase was significantly longer than DURING NMES. In the NMES AT REST phase, the larynx showed anterior–superior movement, but the distance moved was shorter than that of the hyoid bone, and the change was almost the same as the extent of stretching of the laryngeal vestibule. Changes in the laryngeal vestibule and larynx may be related to the anterior–superior movement of the hyoid bone caused by the position of the electrode attachment, which induced traction of the laryngeal vestibule and laryngeal movement by the hyoepiglottic ligament. With saliva swallowing (BEFORE, DURING NMES, and AFTER), the timing of HLx measurement was the same as the timing of the start of hyoid bone elevation in order to explore the automatic feedforward effect of NMES on swallowing movement. For HLx in the AFTER phase, the hyoid bone was positioned anterior–superior because the immediate effect of NMES might increase motor units of the suprahyoid muscle group at the start position of the hyoid bone. However, the motor units related to laryngeal elevation might not have increased as much as the motor units of the suprahyoid muscle group, so it was considered that the HLx in the AFTER phase was extended. Wong et al. reported that the distance of laryngeal elevation adapted according to the HLx before swallowing for the achievement of LVC.22) In the present study, there was no change in the laryngeal elevation distance in aftereffect, but the velocity of laryngeal movement tended to improve (P=0.07, r=0.41). Kendall et al. reported that it is important to measure the timing of LVC and the velocity of laryngeal movement.23)
Nam et al. reported that the intervention effect of NMES improved the velocity of hyoid bone movement,9) and Nagy et al. reported that the velocity of hyoid bone movement was closely related to LVCrt.10) Watts and Dumican reported that LVCrt was shortened as an intervention effect of NMES in healthy subjects.5) In the present study, velocity of hyoid bone movement improved, but change in LVCrt was not statistically significant. This difference between our study and the previous study5) could be caused by an insufficient number of trials in the present study, so an increased number of trials will be necessary to verify our results.
Humbert et al. reported that NMES applied to the infrahyoid muscles prolonged LVCd,24) and Hind et al. reported that effortful swallowing prolonged LVCd.2) In the present study, NMES was applied to the suprahyoid muscle group, but LVCd was extended in the aftereffect. Inamoto et al. reported that LVC persisted for a while after maximal movement of the hyoid bone.25) Therefore, prolongation of LVCd in our current study might have arisen from immediate adaptation of the larynx to the distance moved by the hyoid bone after NMES. In addition, we found a significant dorsal movement of the tongue root during NMES AT REST. We thought that this phenomenon reflected a “posterior ridge of the tongue root” secondary to the contraction of the palatoglossus muscle (which links the soft palate and tongue root) or secondary to significant anterior–superior displacements of the hyoid bone (which might cause deformation of internal tongue muscles via the myoglossus muscle). The palatoglossus muscle is characterized by having muscle spindles in the oral cavity region26) that may be involved in the regulation of swallowing during the transition from the oral phase to the pharyngeal phase.27) Moreover, in the process of swallowing, after the anterior–superior displacements of the hyoid bone,25) the tongue is pulled posteriorly before pharyngeal constriction occurs.28) Given that the mechanism by which NMES affects LVC is yet to elucidated, we thought that it is necessary to verify the effects of stimulation on the internal schema for swallowing movement patterning by feedforward correction.
Considering the above points, NMES for the suprahyoid muscles in the current study can be expected to strengthen the suprahyoid muscles, strengthen the laryngeal elevation muscles, shorten the LVCrt, and prolong the LVCd. In addition, in clinical situations, the risk of pre-swallowing penetration or aspiration because of delayed swallowing movement and post-swallowing penetration or aspiration of hypopharyngeal residue might be reduced.
There are some limitations of this study. First, it was performed on healthy volunteers and only immediate effects were verified. Second, the design of this pilot study included a single phase of “NMES AT REST” before testing the effect of saliva swallowing during NMES. Although the purpose of the insertion was to investigate the effect of “NMES at rest” and to minimize the subject’s exposure to radiation, the preceding single “NMES at rest” might affect the results of saliva swallowing during NMES. Third, the original Ampcare ESP™ protocol recommends using NMES in combination with bringing the chin closer to the chest (chin to chest) with the Restorative Posture Device,11) but we did not completely adopt the original method. The purposes for which we used the device in the current study were to assist with proper cervical alignment, to measure dynamics of oropharyngeal structures, to confirm only the effect of NMES, and to exclude an effect of active contraction of the suprahyoid muscle group. In the future, it will be necessary to assess whether the abovementioned therapeutic effects can be obtained in patients with dysphagia through the application of continuous treatment.
The authors thank the staff of Kirishima Rehabilitation Center of Kagoshima University Hospital for their assistance in this study.
The electrical stimulator used for the intervention was on loan from Ampcare, LLC. The electrodes used in the intervention were provided free of charge by Ito Co., Ltd. No funding, gratuity, or labor was provided by Ampcare or Ito, and the study was conducted without influence from the manufacturers.