2023 Volume 8 Article ID: 20230005
2023 Volume 8 Article ID: 20230005
Recent technological advances in non-invasive brain stimulation (NIBS) have led to the development of therapies for post-stroke upper extremity paralysis. Repetitive transcranial magnetic stimulation (rTMS), a NIBS technique, controls regional activity by non-invasively stimulating selected areas of the cerebral cortex. The therapeutic principle by which rTMS is thought to work is the correction of interhemispheric inhibition imbalances. The guidelines for rTMS for post-stroke upper limb paralysis have graded it as a highly effective treatment, and, based on functional brain imaging and neurophysiological testing, it has been shown to result in progress toward normalization. Our research group has published many reports showing improvement in upper limb function after administration of the NovEl Intervention Using Repetitive TMS and intensive one-to-one therapy (NEURO), demonstrating its safety and efficacy. Based on the findings to date, rTMS should be considered as a treatment strategy based on a functional assessment of the severity of upper extremity paralysis (Fugl-Meyer Assessment), and NEURO should be combined with pharmacotherapy, botulinum treatment, and extracorporeal shockwave therapy to maximize therapeutic effects. In the future, it will be important to establish tailormade treatments in which stimulation frequency and sites are adjusted according to the pathological conditions of interhemispheric imbalance, as revealed by functional brain imaging.
Although the number of patients who suffer from stroke in Japan has been declining over time, it remains high. About 220,000 new strokes and 290,000 recurrent strokes occur annually in Japan,1) and in 2017 there were about 1.115 million stroke patients in Japan.2) Stroke significantly impairs health-related quality of life3) and is the leading cause of disability.4) According to a 2019 survey by Japan’s Ministry of Health, Labour, and Welfare,5) of the major causes of conditions that necessitate long-term care, cerebrovascular disease accounted for 16.1%. Activities of daily living (ADLs) are highly dependent on upper limb function,6) and only 50% of patients who present with upper limb paralysis are able to regain full function of their hand 6 months after onset.7) The clinical importance of rehabilitation therapy for the functional recovery of stroke patients is well known, and rehabilitation must be appropriately performed during the acute, subacute, and chronic phases.
Duncan et al.8) found that improvement in upper limb function in patients with upper limb hemiparesis plateaued 6 months after onset, regardless of severity. However, Jørgensen et al.9) observed that a plateau was reached 6 weeks after onset in mild cases of upper limb hemiparesis, 10 weeks in moderate cases, and 15 weeks in severe cases. Therefore, these studies indicate that there are limitations in treatment based on conventional physical medicine and rehabilitation techniques.
Recently, functional brain imaging techniques and advances in clinical neurophysiology have revealed that an interhemispheric imbalance (IHI) occurs following stroke.10) This finding has led to the recent development of non-invasive brain stimulation (NIBS) techniques. The roles of NIBS in stroke are to increase the excitability of the motor cortex of the lesional hemisphere, correct the IHI, increase the effectiveness of conventional rehabilitation therapy, and reduce residual disability.11) The use of these techniques has led to the development of treatments for post-stroke upper limb hemiparesis, which have been shown to improve upper limb hemiparesis, especially in chronic stroke patients.
This review provides a comprehensive description of the neurophysiological mechanism of repetitive transcranial magnetic stimulation (rTMS) as a NIBS technique for treating post-stroke upper limb hemiparesis. Based on the evidence provided to date, this review also provides tips for clinical practice and summarizes the future prospects for rTMS as a treatment method.
In 1980, Merton and Morton12) succeeded in delivering electrical stimulation to the motor cortex through the scalp in conscious humans, using transcranial electrical stimulation (TES). However, TES was uncomfortable and painful. In 1985, Barker et al.13) applied transcranial magnetic stimulation (TMS) to humans for the first time, as a new cortical stimulation method to replace TES. They generated a magnetic field by passing an electric current through a circular coil and applied it transcranially to the primary motor cortex, succeeding in painlessly recording motor-evoked potential (MEP) in the hand muscles.14)
TMS is a method of stimulating the cerebral cortex by placing an electromagnetic coil on the scalp. The treatment can be adjusted according to the frequency, intensity, and duration of the stimulation. The principle of TMS stimulation of the cerebral cortex is based on Faraday’s law of electromagnetic induction. When an electric current flows through the circular coil, a magnetic field is generated perpendicular to the plane of the coil and passes through the soft tissues and skull to reach the cerebral cortex. It is important to note that the current flowing through the coil must change constantly for the magnetic field to be generated.
Initially, TMS was used as an electrophysiological instrument for measuring MEPs. The technique of rTMS, in which TMS is repeatedly applied, has been shown to induce regional changes in the cerebrum. This led to the application of rTMS to treat upper limb hemiparesis after stroke.
Stimulus frequency is thought to be one of the most important parameters of rTMS. This is because the effects of rTMS on the cerebral cortex vary greatly, depending on the frequency of stimulation. Typically, rTMS above 3 Hz is called high-frequency rTMS and that below 1 Hz is called low-frequency rTMS.15) High-frequency rTMS excites local neural activity in the stimulated area, whereas low-frequency rTMS suppresses it (Fig. 1).16,17,18) A novel form of rTMS is theta burst stimulation (TBS), which is classified into two types: intermittent TBS (iTBS) and continuous TBS (cTBS). Each has the effect of increasing excitability in the lesioned hemisphere and suppressing excitability in the healthy hemisphere.19)
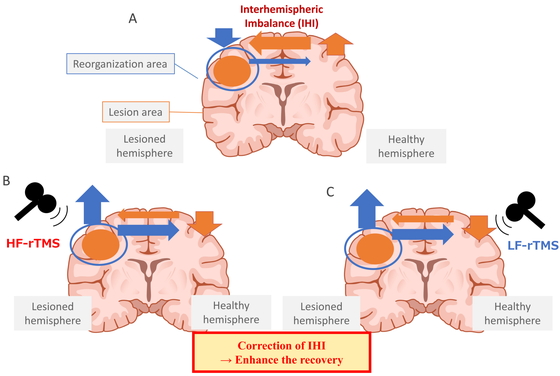
State of IHI and its recovery. Stroke often leads to IHI, in which the interaction between the left and right cerebral hemispheres becomes unbalanced. When a stroke occurs, communication from the lesioned hemisphere to the healthy hemisphere decreases, resulting in IHI and excessive inhibition of the lesioned hemisphere by the healthy hemisphere (A). Activation of the affected hemisphere by high-frequency rTMS (B) or inhibition of the healthy hemisphere by low-frequency rTMS (C) corrects the IHI and restores activity in the lesioned cerebral hemisphere.
The 2020 European guideline published in Clinical Neurophysiology20) rates rTMS treatment for upper limb hemiparesis in stroke patients using a three-level scale: Level A (definitely effective), Level B (probably effective), and Level C (possibly effective). Level A is used for low-frequency stimulation of the intact primary motor cortex to improve upper limb paralysis in the subacute stage (rated as Level B in 2014).21) High-frequency stimulation of the affected primary motor cortex in the subacute phase is rated as Level B (Level C in 2014). Low-frequency stimulation of the intact primary motor cortex to improve upper limb paralysis in the chronic phase is rated Level C (Level B in 2014). The Japan Stroke Society Guideline 2021 for the Treatment of Stroke22,23) states that rTMS or transcranial direct current stimulation (tDCS) may be considered for upper limb dysfunction in the subacute phase and chronic phase with consideration of patient selection and safety (Recommendation Level C, Medium Level of Evidence) (translated from the Japanese version). With respect to pain, rTMS may also be helpful against central pain (Recommendation Level C, Medium Level of Evidence). Furthermore, the guideline describes rTMS treatment for ADL impairment, dysphagia, aphasia, and cognitive dysfunction.
A combination of rTMS and intensive rehabilitation is essential to maximize the recovery of upper extremity function after stroke. Our research group initiated the NovEl intervention using rTMS and intensive one-to-one therapy (NEURO) for upper limb paralysis after stroke in 2008 (Fig. 2).24) The efficacy and safety of NEURO have already been reported,25,26) and the changes that NEURO induces in the brain have been demonstrated using functional brain imaging and neurophysiological testing (Fig. 3).25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49)

NEURO protocol. During the 15-day hospitalization, each subject received daily 40-min low frequency rTMS and 240-min intensive therapeutic exercise (one-to-one training for 120 min and self-exercise for 120 min). The rTMS was directed at the primary motor cortex of the patient’s healthy hemisphere at 2400 pulses a day at a low frequency of 1 Hz. The stimulation intensity was set at 90% of the resting motor threshold for the first dorsal interosseous muscle of the non-paralyzed side.
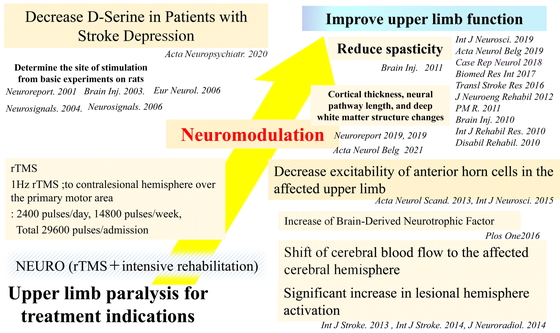
Mechanism of improvement of post-stroke upper limb paralysis using NEURO. NEURO increases cerebral blood flow and activity in the affected hemisphere, increases brain-derived neurotrophic factor, decreases anterior horn cell excitability in the affected upper extremity, and decreases spasticity. These neuromodulations improve upper limb function in patients with post-stroke upper limb paralysis. Other treatment suggestions include changes in cortical thickness, neural pathway length, and deep white matter structure. The level of D-serine should be decreased in patients with post-stroke depression.
The use of rTMS therapy to treat upper extremity paralysis in the acute phase of stroke is considered effective. Swayne et al.50) divided the recovery of motor paralysis into three phases. In the acute phase, the excitability of the remaining corticospinal tracts recovers. During the subacute phase, the excitability of the intercortical network changes. Finally, in the chronic phase, the efficiency of synaptic transmission increases gradually, reaching a plateau at about 6 months after onset. A 2019 meta-analysis of 739 patients from 39 studies reported that rTMS is more effective when performed within 30 days of stroke onset.51) This may be because it is easier to induce brain plasticity, for example, via the reorganization of cortical networks, in the early phases following stroke onset.
High-frequency rTMS may be recommended as the optimal protocol for rTMS treatment in the acute phase. Sasaki et al.52) examined the efficacy of rTMS in acute stroke upper limb paralysis by randomly dividing acute stroke patients with first-episode middle cerebral artery subcortical lesions into three groups. The first group received high-frequency rTMS for the lesional cerebrum, the second group received low-frequency rTMS for the intact cerebrum, and the third group received sham stimulation. All three groups received the stimulation for five consecutive days. The group that received high-frequency rTMS on the lesional side of the cerebrum, which directly activated the impaired corticospinal tracts, showed a significantly greater change in grip strength than the sham stimulation group. Du et al.53) used functional magnetic resonance imaging (fMRI) to examine the effects of rTMS in acute stroke patients. They found that direct activation of the diseased cerebrum with high-frequency rTMS increased cortical activity more than the application of low-frequency rTMS to the intact cerebrum. Furthermore, Sasaki et al.54) performed bilateral rTMS in which high-frequency rTMS to the lesioned cerebrum and low-frequency rTMS to the intact cerebrum were applied simultaneously to patients with acute stroke. This treatment resulted in a significantly greater improvement in the Brunnstrom recovery stage of the upper limb than high-frequency rTMS to the affected cerebral hemisphere. The meta-analysis conducted by Chen et al.55) similarly showed that bilateral rTMS was more effective than unilateral rTMS, and a regimen of 20 rTMS sessions produced greater improvement than a regimen of fewer than 20 sessions in the acute phase. In the future, bilateral treatment using a regimen of more than 20 rTMS sessions should be given in the early stages of the disease to incorporate these innovations.
The 2020 European guidelines20) indicate Level A low-frequency rTMS to the non-lesional primary motor cortex for treatment of upper limb paralysis in the subacute phase. In fact, the recent meta-analysis published in 202255) showed significant effect sizes for recovery of hand function in the subacute phase. In addition, this study also showed that in the subacute phase, stimulation of the affected hemisphere with a 40-session regimen of rTMS was superior to stimulation of the unaffected hemisphere or bilateral hemispheric stimulation in regimens of less than 40 sessions. However, there are some concerns over the application of a uniform rTMS treatment protocol to subacute stroke patients. This is because the status of cortical networks, especially interhemispheric inhibition, in subacute stroke patients varies according to the degree of damage to corticospinal pathways and the stage of recovery.
Hsu et al.56) published a meta-analysis report in 2012 as evidence of the efficacy of rTMS for treating upper limb paralysis after chronic stroke. Eighteen papers published between 2005 and 2011 were included, involving 392 patients who were treated with rTMS. The effect size was 0.66 for the chronic phase. The effect size of low-frequency rTMS was 0.69 and that of high-frequency rTMS was 0.41. The meta-analysis published by Chen et al.55) showed that the effect size of rTMS treatment was 0.38 for the chronic phase. In addition, they reported that stimulation of the unaffected site with a ten-session rTMS regimen produced significant improvement in the chronic phase over the results observed for stimulation of the affected side or bilateral stimulation in regimens of more than ten rTMS sessions. Based on the results of these studies, we recommend ten sessions of low-frequency rTMS to the nonaffected side of the brain in the chronic phase.
A significant number of stroke patients have spasticity in addition to hemiplegia. The manifestation of spasticity varies from case to case. Spasticity may appear during movement and interfere with the execution of activities. Treatment with rTMS modulates excitability by stimulating the cerebral cortex, facilitating information signals between cortical cells and spinal cord anterior horn cells. This reduces both the hyperexcitability of the anterior horn cells and the sensitivity of muscle spindles, resulting in reduced spasticity. Both high-frequency rTMS to the lesioned cerebrum and low-frequency rTMS to the intact cerebrum increase neural activity in the lesioned cerebrum and have antispastic effects.57,58) Kakuda et al.46) evaluated upper limb motor function and the degree of upper limb spasticity before and after treatment with low-frequency rTMS and intensive therapeutic exercise for 15 days in patients with post-stroke upper limb paraplegia and spasticity. Based on the Fugl-Meyer Assessment (FMA) and the Wolf Motor Function Test (WMFT), the patients exhibited improvement in upper limb motor function on the paralyzed side. A decrease in muscle tonus in the flexor muscles of the paralyzed upper limb was also confirmed by reduced Modified Ashworth Scale scores. Kondo et al.47,59) also reported that low-frequency rTMS to the intact cerebellum improved the pathological hyperexcitability of motor neurons in the paralyzed upper extremity. These results indicate that low-frequency rTMS to the healthy cerebellum has an antispasmodic effect.
Intracranial metal, intracardiac catheters, and cardiac pacemakers are absolute contraindications for rTMS. Relative contraindications are pregnancy, infanthood, heart disease, implanted medication pumps, family history of epilepsy, seizures, and magnetic dentures.60) Seizures are considered one of the most harmful side effects of rTMS. With respect to this side effect, Kakuda et al.26) reported on the safety of using rTMS in the clinical setting to treat upper limb hemiparesis. Their multicenter study involved 1725 patients who had not suffered a seizure for at least 1 year and had no seizure-producing abnormalities on electroencephalography. Low-frequency stimulation of the healthy cerebral hemisphere did not produce a seizure, which is considered the most harmful side effect, and there was no worsening of upper limb paralysis. Minor adverse events that did not require treatment occurred in 1.3% (22) of patients. These were dizziness in 9 patients, discomfort at the site of stimulation in 7 patients, and headache in 6 patients. None of these events led to the discontinuation of treatment with rTMS. Training intensity had to be reduced for 1.5% (26) of patients after they started treatment. None of the patients experienced adverse events after discharge.
We believe that combining rTMS therapy with intensive rehabilitation therapy, rather than using it as a standalone treatment, is essential for effectively improving upper limb function. Our group has conducted many NEURO studies that have confirmed the effectiveness of this approach. As mentioned above, Kakuda et al.26) conducted a large open-label study to demonstrate the efficacy and safety of NEURO for improving upper extremity function. Niimi et al.27) demonstrated that NEURO activates the brain-derived neurotrophic factor (BDNF) pathway by measuring serum levels of BDNF, proBDNF, and matrix metalloproteinase 9 (MMP9), which are thought to play important roles in brain damage. Using fMRI, Yamada et al.28) showed that improvements in FMA and WMFT scores resulting from NEURO treatment were correlated with activation of the lesional hemisphere. Takekawa et al.29) used single-photon emission computed tomography (SPECT) to show that the improvement in FMA scores following NEURO was correlated with the laterality index of that hemisphere. These results indicate that functional reorganization in the injured hemisphere and the residual area surrounding the injured site play an essential role, indicating the neurophysiological efficacy of NEURO treatment. Abo et al.48) conducted a randomized controlled trial comparing constraint-induced movement therapy (CIMT) and NEURO and observed significant improvements in FMA and WMFT scores as a result of both treatments. However, the effect of NEURO was significantly greater than that of CIMT. They also reported that rTMS imposed a lower burden on patients and trainers than CIMT. Furthermore, Yamada et al.61) conducted an observational study of the respective treatment effects of NEURO and therapeutic exercise alone. They reported that both FMA and WMFT scores improved significantly more strongly in the NEURO group than in the therapeutic exercise group.
Combining rTMS with other therapies is also an important strategy for improving upper limb paralysis. Although there is limited research on the combination of rTMS with botulinum toxin therapy, one case series has reported improvement in spasticity after treatment with this combination.62) As noted above, rTMS alone has an antispastic effect.46) Therefore, in cases of severe spasticity, botulinum therapy combined with rTMS is a highly effective way to achieve improvement in voluntary movement and spasticity while reducing tension in the proximal muscles of the upper extremity. Recently, extracorporeal shockwave therapy (ESWT) has attracted attention as a new treatment strategy for spasticity. It was first used in urology and later in orthopedics, where it has been shown to have analgesic and muscle-relaxing effects.63,64) The use of ESWT for the treatment of upper and lower limb spasticity in stroke and cerebral palsy has been shown to be effective in adults and children.65,66) Although the mechanism is still largely unknown, studies using rat models have reported that ESWT has a destructive effect on nerve endings that is confined to the posterior synapses.67) We believe that the combination of ESWT and rTMS may yield additional therapeutic effects.
This section discusses the target population for rTMS therapy. Hamaguchi et al.68) classified 1254 patients treated with NEURO into three groups, based on the pattern of improvement in their upper limb paralysis as indicated by FMA scores. Patients with FMA scores that improved by 10 or greater in response to NEURO treatment were classified as hyper-responders. Patients with FMA scores that improved by 5–10 were classified as responders, and those with FMA scores that improved by 5 or less were classified as non-responders. Most patients with mild disability exhibited some improvement, but it was unlikely to be significant in terms of change in the FMA score. In contrast, most of the hyper-responders and responders (i.e., those who experienced high treatment efficacy) had moderate pre-treatment FMA scores. We therefore suggest that recovery of upper limb motor paralysis in response to NEURO treatment is more likely in patients with moderate pre-treatment FMA scores. There is also a report that shows no difference in the therapeutic effect of NEURO treatment between cerebral infarction and cerebral haemorrhage.69) Based on these findings, it is necessary to consider the potential for recovery with each treatment method70,71,72) and combine them carefully to maximize the therapeutic effect. A future treatment strategy to improve upper limb function after stroke may be to compile a list of treatment selection criteria that would be assessed based on an evaluation of stroke paralysis (Fig. 4).

An example of criteria for treatment selection based on the stroke upper extremity paralysis assessment (FMA score). If the FMA score is low (≤ 22)and paralysis of the upper limb extremities is severe, then botulinum treatment, repetitive peripheral magnetic stimulation (rPMS) treatment, and ESWT are used to reduce muscle tone in the proximal upper extremity and improve upper extremity motor function. If upper extremity function is moderate (FMA score of 23–52), NEURO is highly effective and recommended. In this case, we recommend combining botulinum therapy, rPMS, and ESWT. If the restriction of upper extremity function is mild (≥53), then NEURO is recommended.
The most important consideration when performing rTMS early in the stroke process is to manage the risk of epilepsy. When performing high-frequency rTMS, the risk of inducing epileptic seizures via the forcible activation of the lesional cerebral cortex must be considered. Post-stroke epilepsy is broadly classified into early and late seizures, according to the time of onset. The pathogenesis of the two types of epilepsy differs. Early seizures, which occur within 1–2 weeks of onset, are caused by local metabolic changes in the brain following the stroke and direct stimulation of the cerebral cortex by blood degradation products, which lower the threshold for epileptic seizures.73) In contrast, late seizures are thought to occur as a result of the formation of epileptogenic foci. It has been reported that the complication of epilepsy occurs in approximately 10% of stroke patients.74) In addition, post-stroke epilepsy often occurs without seizures, especially in the early type, and may develop into nonconvulsive status epilepticus (NCSE), making EEG monitoring important in determining the indication for rTMS.75) Recently, arterial spin labeling (ASL) has become available as an MRI-based method for measuring cerebral blood flow.76) This technique allows the efficient estimation of cerebral blood flow using radiofrequency pulses without contrast agents or radiation tracers, making it less invasive than conventional SPECT. During epileptic seizures, increases in blood flow in the epileptic foci and the areas that are associated with seizure syndromes can be visually identified. This approach is considered useful for identifying epilepsy prior to rTMS treatment.
In recent years, research into the use of rTMS to treat depression has progressed. High-frequency rTMS to the left prefrontal cortex is the standard stimulation condition for treatment-resistant depression. It has been reported that rTMS treatment results in significant improvements in depression symptoms.77) Kito et al.78,79) suggested that rTMS modifies the neural networks in the left dorsolateral prefrontal cortex, posterior cingulate gyrus, and left dorsolateral prefrontal limbic regions. In stroke, the prevalence of post-stroke depression is high, with 31% of patients experiencing depression within 5 years of stroke onset.80) Therefore, a substantial percentage of patients experience the complication of post-stroke depression during the course of rTMS treatment for upper extremity paralysis. When administering rTMS treatment to patients presenting with symptoms of depression, there is a theoretical risk of worsening their depressive symptoms with low-frequency rTMS treatment to the left cerebral hemisphere or with high-frequency rTMS treatment to the right cerebral hemisphere.
The optimal protocol for rTMS treatment should be established by considering not only the excitability of the corticospinal tracts, but also that of the intercortical network. It is therefore necessary to evaluate the degree of IHI and cortical activity as well as the stage of disease. Tamashiro et al.49) used functional near-infrared spectroscopy (fNIRS) to examine the relationship between the asymmetry in brain activity before rTMS treatment and the effects of low-frequency rTMS on upper limb motor function. They found that motor recovery was greater in patients in whom the non-lesional hemisphere was dominant than in those in whom the lesioned hemisphere was dominant. There was also a significant correlation between brain asymmetry before low-frequency rTMS and the extent of improvement in upper limb motor function. This suggests that motor recovery after treatment with low-frequency rTMS can be predicted by brain asymmetry, as assessed by using fNIRS.
As mentioned above, rTMS treatment is considered effective when applied as bilateral treatment to both cerebral hemispheres in the acute phase, as high-frequency rTMS to the affected hemisphere in the subacute phase, and as low-frequency rTMS to the healthy cerebral hemisphere in the chronic phase.47,51) These differences in the recommended protocols may reflect the gradual change in the IHI between the affected and healthy hemispheres that occur during the transition from the acute phase to the chronic phase. Furthermore, the emergence of the IHI is not the same in all post-stroke patients and has been shown to be associated with compensatory movements.28,81) Therefore, rTMS treatment should be tailored to individuals based on functional brain imaging rather than following a uniform treatment protocol that is based on the disease phase or the severity of paralysis. In fact, a study of post-stroke aphasia patients showed that low-frequency rTMS applied to a region of the contralateral hemisphere homologous to the region activated while performing a language task (identified by fMRI) improved language function.82,83) However, further prospective studies are needed to confirm the usefulness of neuroimaging-based rTMS therapy for upper extremity hemiplegia.
In addition, rTMS is expected to be introduced into clinical practice, especially for treatment of the acute and subacute phases. However, rTMS is not covered by the Japanese medical insurance system, and there are many challenges associated with its implementation, such as equipment installation and the allocation of personnel. Nevertheless, it is expected that rTMS will become more widely administered in acute and subacute settings in the future.
We thank the staff of the Department of Rehabilitation Medicine at the Jikei University School of Medicine. We also thank Uni-edit (https://uni-edit.net/) for editing and proofreading this manuscript.
The authors declare no conflict of interest.