ABSTRACT
Background: Task-specific dystonia (TSD) confined to the lower extremities
(LE) is relatively rare. This report describes dystonia confined to the LE only during
forward walking. This case required careful neurological and diagnostic assessment because
the patient was taking several neuropsychiatric drugs that cause symptomatic dystonia,
such as aripiprazole (ARP).
Case: A 53-year-old man visited our university hospital with a complaint of
abnormalities in the LE that appeared only during walking. Neurological examinations other
than walking were normal. Brain magnetic resonance imaging revealed meningioma in the
right sphenoid ridge. The patient had been treated for depression with neuropsychiatric
medications for a long time, and his abnormal gait appeared about 2 years after additional
administration of ARP. After the meningioma was removed, his symptoms remained. Surface
electromyography showed dystonia in both LE during forward walking, although his abnormal
gait appeared to be accompanied by spasticity. The patient was tentatively diagnosed with
tardive dystonia (TD). Although dystonia did not disappear clinically, it was alleviated
after discontinuing ARP. Administration of trihexyphenidyl hydrochloride and concomitant
rehabilitation improved his dystonia until return to work, but some residual gait
abnormalities remained.
Discussion: We report an unusual case of TD with task specificity confined
to the LE. The TD was induced by the administration of ARP in combination with multiple
psychotropic medications. Careful consideration was required for clinical diagnosis,
rehabilitation, and assessment of its relevance to TSD.
INTRODUCTION
Symptomatic dystonia includes diverse etiologies, with one representative instance being
tardive dystonia (TD) induced by neuroleptics with dopaminergic blocking action. Adult-onset
TD usually involves both craniocervical dystonia and segmental dystonia.1) Here, we report a patient who was
hospitalized for the purpose of surgery for meningioma. Prior to hospitalization, the
patient, who was also being treated for depression, showed TD in the lower extremities (LE)
after additional administration of ARP (ABILIFY®, Otsuka Pharmaceutical), which is a partial
dopamine (DA) agonist of the DA D2 receptor. Dystonia was ameliorated after ARP cessation,
which suggested that it might have been induced by ARP. TD confined to the LE occurs at a
low prevalence; in this case, the dystonia only appeared during walking. These findings
indicate that the TD may have resembled task-specific dystonia (TSD). Although previous
reports have described tardive syndrome that was attributed to ARP, dystonia appearing in
the LE has not been reported.2)
We report that TSD, which is similar to spastic gait and is localized to the LE during
walking, is rare and should be noted for rehabilitation intervention.
CASE
A 53-year-old man was receiving long-term treatment for depression with a regimen of
neuropsychiatric drugs that included lithium carbonate (Limas®, Taisho Pharmaceutical,
Japan), lorazepam (Wypax®, Pfizer Japan, Japan), flunitrazepam (Silece®, Eisai, Japan),
fluvoxamine (Luvox®, Astellas Pharma, Japan), clonazepam (Landsen®, Sumitomo Pharma, Japan),
and brotizolam (Lendormin®, Boehringer Ingelheim, Germany). Approximately 6 months after
increasing the daily dose of ARP from 3 mg to 6 mg, he complained of trouble walking on
planar floors and soon after of difficulties when pushing off the ground (Fig. 1). The patient visited the Department of
Neurosurgery of our hospital on March 25, 2019 (Fig.
1). He had no history of perinatal or developmental disorders, general medical
disease, head injury, drug abuse, or preceding abnormal involuntary movements. The patient
was able to walk and perform daily activities independently. He was also able to commute to
work and had no history of falls. Brain magnetic resonance imaging (MRI; Ingenia Provida
1.5T, Philips, USA) indicated a right sphenoid ridge meningioma. He was admitted to our
hospital on April 16, 2019. The brain tumor (BT) was removed by neurosurgery on April 17,
2019 (Fig. 2A,B).


Despite complete removal of the BT, the disturbance of ankle movement during walking
persisted. The day after BT removal, the patient was referred to the Rehabilitation
Department. During the stance phase, he showed difficulties of right ankle inversion,
plantar flexion, and dorsiflexion (Fig. 3A). This
disturbance of ankle movement appeared only during gait. He could move his ankle without any
difficulties while sitting, pedaling, and even during backward walking. At the start of
rehabilitation on April 18, 2019 (Fig. 1), the
muscle strength of the bilateral iliopsoas quadriceps, tibialis anterior, and gastrocnemius
was almost 4 with manual muscle testing. Trunk balance was within normal limits, with a
standing retention time of more than 10 s on each side in the tandem limb position with open
eyes. He was prescribed a regimen of physical therapy for 40 min a day. Blood pressure
during orthostatic movements was stable, and no discomfort or dizziness was noted during
sitting, standing, or walking. At the start of gait training, his physical therapist noted
an abnormal gait with the involvement of both LE. Dystonia was suspected by the physiatrist
because an abnormal muscle tone in the LE was induced only during walking. Abnormal muscle
tone in the LE did not appear in backward or lateral gait. However, there was a risk of
tripping and falling because of decreased stability during walking, probably caused by the
combined effects of bedrest after surgery and the abnormal gait. Therefore, consideration
was given to rehabilitation aimed at restoring LE muscle strength and improving coordinated
movements of the LE, especially the ankle joint, without inducing TSD. At the same time, the
ward nurses were instructed to assist the patient in walking by grasping the ipsilateral
axilla with the unilateral upper limb to prevent the ankle joints from crossing during
walking.

Rehabilitation specifically for TSD confined to the LE has not yet been established.
Therefore, we attempted to understand the characteristics of the patient’s motor abilities
to avoid TSD in this case, considering the task specificity of forward walking, and to find
sensory motor reorganization, such as finding sensory tricks, based on a study of a small
number of cases of spasticity and spastic dystonia in the past.1,2) In this case, TSD was not induced by lateral or backward
walking, nor by supine bicycle pedaling, and these were actively employed. In addition,
stretching and isometric strength training did not induce TSD, and these were performed in
parallel. Given that sensory tricks may be effective in treating dystonia, we tested
measures that included the use of shoe soles with fine uneven special marks to stimulate the
plantar area, binding of the LE, and application of vibration to the front part of the LE.
However, we were unable to find a specific tactile stimulus that was effective for his
dystonia. On April 26, 2019 (the last day of rehabilitation), the patient had a Barthel
Index (BI) of 95 points, 5 of which were for bathing, and, although the TSD remained, the
patient improved to the point where he was able to receive home care (Fig. 1). This improvement was attributed to a short but continuous
rehabilitation program that avoided TSD.
Upon discharge from hospital, the patient was instructed in home exercises. The home
exercises included lifting the hips in the supine position and standing on tiptoe while
supporting the body with one hand in the standing position, thereby maintaining the range of
motion and muscle strength in the LE without inducing TSD. The patient was also instructed
to perform stretching exercises in a sitting position with towels placed over both ankle
joints to extend the knees and back in a forward bending position, and balance exercises in
a standing position with both upper limbs supporting the trunk and holding the posture while
extending the bilateral Achilles tendons. Imaging of the dopamine transporter by
123I-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)tropane single-photon
emission computed tomography (123I-FP-CIT-SPECT; Symbia S, Siemens Medical
Solutions, USA) on May 9, 2019, showed that dopamine transporter binding capacity was
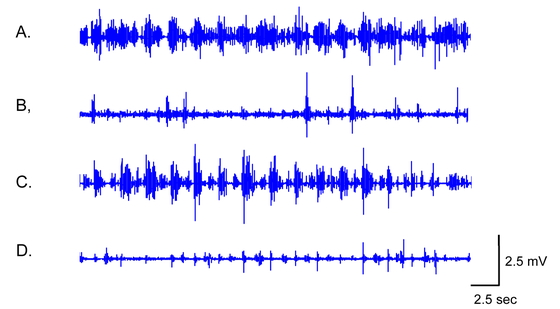
functionally normal (specific binding ratio, SBR: right=5.10, left=5.47; Fig. 2C). Surface electromyography (EMG; Trigno Avanti
Platform; Delsys, Natick, MA, USA) showed the findings of dystonia in his LE (Fig. 4).
At the first post-discharge rehabilitation outpatient visit on June 29, 2019, approximately
2 months after cessation of ARP on May 11, 2019, the BI showed improvement with a full score
of 100, while TSD during forward walking remained but showed improvement (Fig. 3B). After discharge, his dystonia still appeared
crus predominant in both LE immediately after the start of forward walking. Therefore, he
was prescribed 6 mg of trihexyphenidyl hydrochloride (TH) per day at a neurology outpatient
clinic on September 11, 2019, after which his gait improved further, and he was able to
return to work. The combination of rehabilitation and medication was effective in improving
TSD as well as both muscle weakness and incoordination in LE. Written informed consent was
obtained from the patient for publication of this report and the accompanying images.
DISCUSSION
The dystonia in this case appearing in the LE was activated only during walking and emerged
after additional ARP administration during long-term neuropsychiatric treatment.
Neuroleptics can induce movement disorders such as parkinsonism, akathisia, dyskinesia, and
dystonia. The pathophysiology is usually considered to result from DA D2 receptor blockade
during long-term neuroleptic exposure.
Dystonia caused by neuroleptics, termed TD, is less common than other tardive syndromes
such as parkinsonism and choreic dyskinesia. Burke et al.3) proposed criteria for the diagnosis of drug-induced dystonia
and TD in 1982. According to these authors, the patient must have received neuroleptic
treatment for at least 3 months. However, another report stated that one-fifth of the
examined cases had been treated for less than one year, and half of the cases for more than
5 years.4) The frequency of TD
varies from 0.4% to 21.6%,4)
with a mean prevalence of 5.3%. There are only 14 reported cases worldwide of TSD localized
to the LE during intense exercise, including walking.5) However, a case of drug-induced TSD confined to the
bilateral LE, as in the case reported here, has never been observed. Katz et al. reported
that all seven cases of TSD localized to the LE were unilateral.5) The prevalence of primary focal dystonia in adults,
including the LE, is reported to represent only 0.7% of all adult-onset primary
dystonia.6) Therefore, cases
of dystonia confined to the LE with delayed onset and task specificity appear to be
extremely rare.
The patient’s dystonia gradually developed after additional ARP administration;
consequently, gait abnormalities improved after ARP cessation, which suggested drug-induced
TD. ARP, a partial DA agonist at the DA D2 receptor, is thought to activate presynaptic DA
D2 receptors to cause TD.1)
However, the pathophysiology and pathogenesis of TD remain unclear, although several
hypotheses have been proposed. This pathological condition is generally thought to result
from postsynaptic hypersensitivity caused by persistent inhibition of DA
neurotransmission.7)
Inhibition of postsynaptic DA neurotransmission, caused by the agonistic effects of ARP on
presynaptic dopaminergic nerves, may result in postsynaptic DA receptor antagonism by the
usual antipsychotic agents involved in TD. We speculate that the specific effects of ARPs,
which differ from those associated with common postsynaptic DA receptor antagonism by
antipsychotics involved in TD, may have contributed to the development of TSD in this case.
Peña et al. reported eight patients with tardive syndromes caused by ARP, with
oro-bucco-lingual stereotypy being the most frequent.2) In our case, however, the dystonia only appeared in the LE
during walking. This finding is similar to isolated lower limb dystonia, which is usually
associated with Parkinson’s disease, peripheral trauma, and other brain lesions including
cerebrovascular disease,8) but
is rarely induced by neuroleptics.1) Schneider et al. reported a series of patients with isolated
lower limb dystonia.8) They
observed isolated lower limb dystonia with plantar flexion of every toe and inversion of the
foot, which were exaggerated during walking or standing, similar to our patient. None of
their patients showed drug-induced dystonia.8)
In the present study, the 123I-FP-CIT SPECT was functionally normal, which
indicated that the patient did not have Parkinson’s disease. Abnormal sensorimotor
plasticity and loss of cortical inhibition have been implicated in the etiology of
TSD.9,10) However, hypotheses of the
condition cannot explain why these general changes appear only in certain parts of the body
and not in unaffected regions.9)
A recent broad definition of TSD included loss of motor control confined to a specific motor
skill; moreover, task specificity in focal dystonia may not be limited to skilled actions,
with focal TSD occurring in activities that are relatively automatic.11) An investigation using
11C-raclopride positron emission tomography revealed that reduced striatum DA
D2/D3 receptor levels might play important roles in TSD.12) Therefore, ARP, a DA receptor partial agonist, may be
relevant to the cause of this patient’s TSD. Anticholinergic medications (AM), such as TH,
are commonly used to treat dystonia.13) AM is administered based on the speculative theory of
imbalance between dopaminergic and cholinergic neurotransmission. According to this theory,
imbalance results from the blockage of dopaminergic neurotransmission caused by
antipsychotic drugs, which results in cholinergic dominance in the striatum. After cessation
of ARP in the patient, dystonia was alleviated, but his daily activities remained
restricted. Therefore, after discharge from the hospital, TH was prescribed in the
outpatient clinic of the Neurology Department of this hospital and was found to be effective
(Fig. 1).
Although rehabilitation for focal dystonia including TSD has not been established, some
findings are showing the effectiveness of drug treatment and concomitant use with botulinum
toxin.14) There is one
report of ankle foot orthosis applied to a patient with TSD,5) but this therapeutic approach was adopted because
the patient presented a mild deformity of the ankle joint on the affected side that had been
caused by painful dystonia. This scenario did not apply to our patient, who was able to
perform walking exercises without the use of orthoses. If the patient had suffered prolonged
pain, a lower limb orthosis might have been considered for safe ambulation. In the future,
guidelines for orthotics in TSD should be carefully considered. Because abnormal
sensorimotor reorganization for specific movements has been postulated as a
pathophysiological mechanism of TSD, proposed rehabilitation methods have attempted to avoid
excessive sensory input and have attempted to reorganize sensory information
processing.15) Strength
training, stretching, relaxation, and postural exercises incorporated in this patient were
considered potentially effective for TSD as learning-based motor-sensory exercises to
reorganize sensory information processing,16) but no effective results were obtained. Excessive muscle
strength training and joint range of motion training in the affected area may cause an
increase in sensory input and exacerbate dystonia. However, recovery of muscle strength in
the LE and improvement of BI were obtained without aggravation of TSD, suggesting that
inpatient rehabilitation was beneficial.
To date, rehabilitation guidelines for TSD have not been established, possibly because very
few cases of TSD are confined to the LE and no large-scale cohort studies have been
conducted. Therefore, it has been difficult to construct evidence-based rehabilitation
treatment strategies for TSD. In the present case, we attempted to understand the
characteristics of the patient’s motor ability to avoid TSD, considering the task
specificity of forward walking, and to practice walking using methods to modify plantar
sensation, such as sole shape. We observed that TSD was not induced by walking laterally or
backward, nor by bicycle pedaling in the supine position. Furthermore, stretch exercises and
isometric strength training did not elicit TSD, and these were used for walking
exercises.
Rehabilitation for dystonia has been attempted in the past, and rehabilitation using
various sensory stimuli has been shown to be useful for focal dystonia, such as Musician’s
cramp, albeit in small studies, and positive results have been suggested.17,18) However, in this case, the use of vibration of the
LE and the use of soles that stimulate the sole did not provide any benefit for TSD of the
LE during forward walking. Although the reason is not clear, a previous study of patients
with writer’s cramp described an intensive 8-week period during which movements and work
exercises were performed to avoid movements that induce dystonia.19) Therefore, in the present case, the
hospitalization may have been too short to provide a useful rehabilitation effect. In
addition, the home exercise program, which focused primarily on muscle weakness through
stretching and isometric strength training, may have been inadequate for the rehabilitation
of TSD. As a limitation in this study, noninvasive neuromodulation therapies such as
transcranial magnetic stimulation and botulinum toxin injections, which have been reported
to improve TSD by normalizing brain excitability through sensorimotor reorganization, should
have been considered as rehabilitation treatment options for TSD.16) Therefore, considering the difficulty of
rehabilitating TSD and the difficulty of avoiding forward walking in daily life, it may have
been beneficial for the patient to have been transferred to a convalescent hospital to
continue rehabilitation for a period of 2 months or longer.
ACKNOWLEDGMENTS
We thank Dr Peter Neri, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/)
for editing a draft of this manuscript. This case was presented at the 18th Japanese
Association of Rehabilitation Medicine Kanto Regional Meeting on September 29, 2019.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1. Savitt D, Jankovic J: Tardive syndromes. J Neurol
Sci 2018;389:35–42. PMID:29506749, DOI:10.1016/j.jns.2018.02.005
- 2. Peña MS, Yaltho TC, Jankovic J: Tardive
dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord
2011;26:147–152. PMID:20818603, DOI:10.1002/mds.23402
- 3. Burke RE, Fahn S, Jankovic J, Marsden CD, Lang
AE, Gollomp S, Ilson J: Tardive dystonia: late-onset and persistent dystonia caused by
antipsychotic drugs. Neurology 1982;32:1335–1346. PMID:6128697,
DOI:10.1212/WNL.32.12.1335
- 4. Kang UJ, Burke RE, Fahn S: Tardive dystonia. In:
Fahn S, Marsden CD, Calne DB, editors. Advances in Neurology. Dystonia 2. Vol. 50. New
York: Raven Press; 1988. pp. 415–429.
- 5. Katz M, Byl NN, San Luciano M, Ostrem JL: Focal
task-specific lower extremity dystonia associated with intense repetitive exercise: a case
series. Parkinsonism Relat Disord 2013;19:1033–1038. PMID:23932354,
DOI:10.1016/j.parkreldis.2013.07.013
- 6. Martino D, Macerollo A, Abbruzzese G, Bentivoglio
AR, Berardelli A, Esposito M, Fabbrini G, Girlanda P, Guidubaldi A, Liguori R, Liuzzi D,
Marinelli L, Morgante F, Sabetta A, Santoro L, Defazio G: Lower limb involvement in
adult-onset primary dystonia: frequency and clinical features. Eur J Neurol
2010;17:242–246. PMID:19765051, DOI:10.1111/j.1468-1331.2009.02781.x
- 7. LeWitt PA: Dystonia caused by drugs. In: Tsui
JKC, King J, Calne DB, editors. Handbook of Dystonia. New York: Marcel Dekker; 1995. pp.
227–240.
- 8. Schneider SA, Edwards MJ, Grill SE, Goldstein S,
Kanchana S, Quinn NP, Bhatia KP, Hallett M, Reich SG: Adult-onset primary lower limb
dystonia. Mov Disord 2006;21:767–771. PMID:16456826,
DOI:10.1002/mds.20794
- 9. Hallett M: Neurophysiology of dystonia: the role
of inhibition. Neurobiol Dis 2011;42:177–184. PMID:20817092,
DOI:10.1016/j.nbd.2010.08.025
- 10. Quartarone A, Morgante F, Sant’Angelo A, Rizzo V,
Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P: Abnormal plasticity of
sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol
Neurosurg Psychiatry 2008;79:985–990. PMID:17634214,
DOI:10.1136/jnnp.2007.121632
- 11. Lo SE, Frucht SJ: Is focal task-specific dystonia
limited to the hand and face? Mov Disord 2007;22:1009–1011. PMID:17571347,
DOI:10.1002/mds.21141
- 12. Berman BD, Hallett M, Herscovitch P, Simonyan K:
Striatal dopaminergic dysfunction at rest and during task performance in writer’s cramp.
Brain 2013;136:3645–3658. PMID:24148273, DOI:10.1093/brain/awt282
- 13. Sadnicka A, Kassavetis P, Pareés I, Meppelink AM,
Butler K, Edwards M: Task-specific dystonia: pathophysiology and management. J Neurol
Neurosurg Psychiatry 2016;87:968–974. PMID:26818730,
DOI:10.1136/jnnp-2015-311298
- 14. Sheean G: Restoring balance in focal limb
dystonia with botulinum toxin. Disabil Rehabil 2007;29:1778–1788. PMID:18033603,
DOI:10.1080/09638280701568742
- 15. Sadnicka A, Rosset-Llobet J: A motor control
model of task-specific dystonia and its rehabilitation. Prog Brain Res 2019;249:269–283.
PMID:31325986, DOI:10.1016/bs.pbr.2019.04.011
- 16. Hautekiet A, Raes K, Geers S, Santens P, Oostra
K: Evidence of rehabilitation therapy in task-specific focal dystonia: a systematic
review. Eur J Phys Rehabil Med 2021;57:710–719. PMID:33619945,
DOI:10.23736/S1973-9087.21.06677-6
- 17. Sadnicka A, Rosset-Llobet J: A motor control model
of task-specific dystonia and its rehabilitation. Prog Brain Res 2019;249:269–283.
PMID:31325986, DOI:10.1016/bs.pbr.2019.04.011
- 18. Zeuner KE, Molloy FM: Abnormal reorganization in
focal hand dystonia—sensory and motor training programs to retrain cortical function.
NeuroRehabilitation 2008;23:43–53. PMID:18356588,
DOI:10.3233/NRE-2008-23105
- 19. Byl NN, Archer ES, McKenzie A: Focal hand
dystonia: effectiveness of a home program of fitness and learning-based sensorimotor and
memory training. J Hand Ther 2009;22:183–198. PMID:19285832,
DOI:10.1016/j.jht.2008.12.003




