2023 Volume 8 Article ID: 20230019
2023 Volume 8 Article ID: 20230019
Background: Carnitine is a vital human nutrient. Although there are many reports on carnitine deficiency, most studies have been conducted on children, patients with severe mental and physical disabilities, epileptic patients, patients with liver cirrhosis, and dialysis patients. To the best of our knowledge, there are no reports on carnitine administration for disorders of consciousness after stroke. We report two such cases in which carnitine administration improved disorders of consciousness.
Cases: Case 1 was a woman in her sixties who was admitted to our rehabilitation center 4 months after the onset of subarachnoid hemorrhage. After admission, her disorders of consciousness worsened even though she was actively undergoing rehabilitation. Suspecting carnitine deficiency, we administered 1500 mg/day of L-carnitine, which resulted in improvement of her disorders of consciousness and disappearance of symptoms such as convulsions. Case 2 was a man in his thirties who was admitted to our rehabilitation center 5 months after the onset of cerebral hemorrhage. During active rehabilitation, he suffered worsening disorders of consciousness, convulsions, and cramps. We found carnitine deficiency with a blood carnitine concentration of 21 mg/dL, so we administered 1500 mg/day of L-carnitine; symptoms of disorders of consciousness and convulsions then improved.
Discussion: It is possible that carnitine deficiency has been overlooked in some patients in rehabilitation wards, and measurement of ammonia might facilitate its detection. Because carnitine deficiency can interfere with active rehabilitation, nutritional management with attention to carnitine deficiency could be important during rehabilitation.
Carnitine is a nutrient that is involved in various metabolic mechanisms, including lipid metabolism, and is particularly necessary for the transport of long-chain fatty acids into the inner mitochondrial membrane.1,2,3) Carnitine deficiency can be divided into primary carnitine deficiency, which is caused by a genetic abnormality in the carnitine transporter that reduces carnitine uptake in muscle cells and renal tubules, and secondary carnitine deficiency, which is caused by other factors.4,5) Symptoms include hyperammonemia, seizures, and disorders of consciousness. Although many studies on carnitine deficiency have been reported, most have been conducted on children, people with severe mental and physical disabilities, epilepsy patients, patients with liver cirrhosis, and dialysis patients; there have been few reports of studies of carnitine deficiency affecting rehabilitation treatment of patients in rehabilitation wards.6,7) In particular, to our knowledge, there are no reports regarding carnitine administration for disorders of consciousness in patients after stroke. Here, we report two cases of patients with subacute stroke who showed improved outcomes for disorders of consciousness after administration of carnitine during hospitalization in a rehabilitation ward. This study was approved by the Ethics Committee of Kagoshima University Hospital (Approval No. 190269 Epidemic). Informed consent for the study was obtained by the opt-out method.
A woman in her sixties was admitted to an acute care hospital for subarachnoid hemorrhage. During the course of the disease, a tracheostomy was performed because of severe dysphagia, and nasal tube feeding was performed. She also received a ventriculoperitoneal shunt because of hydrocephalus. After initial treatment, the patient’s general condition stabilized, and she was admitted to our rehabilitation center 4 months after the onset. Physical examination on admission showed the following: height, 153 cm; body weight, 46 kg; body mass index, 19.6; body temperature, 37.0 °C; blood pressure, 110/70 mmHg. The level of the patient’s consciousness was [E4 V (T) M6] on the Glasgow Coma Scale (GCS). She required full assistance with activities of daily living (ADL), but when we instructed her to open and close her eyes, she was able to comply. As a result of intensive rehabilitation, she was able to maintain a sitting position and move about 20 m by herself using a wheelchair. However, this activity gradually became more difficult after about 4 weeks of hospitalization. Furthermore, her rating on the GCS deteriorated to [E3 V (T) M5] and myoclonus and generalized tonic-clonic convulsions were observed. Computed tomography (CT) of the head showed no worsening of hydrocephalus, but blood tests revealed hyperammonemia with a blood ammonia level of 105 µg/dL. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and blood glucose levels were within normal limits, and urine ketones were negative. We therefore made a differential diagnosis of hyperammonemia. No congenital metabolic disease had been previously noted, and other metabolic and liver diseases were absent based on blood and urine tests. CT showed no abnormalities in the morphology of her liver. We assessed the patient for increased ammonia production by reviewing the amount of protein in the diet and strengthening defecation control, but the blood ammonia level did not improve; on the contrary, it worsened to 142 µg/dL. Table 1 shows blood test data from this period. In addition, the patient had no previous history of epilepsy, but at this time she began to have generalized seizures. We started antiepileptic drugs, but neither the disorders of consciousness nor the convulsions improved. Consultation with a hepatologist revealed normal liver morphology and no portosystemic shunt. By exclusion, carnitine deficiency was suspected as the cause of nonhepatic hyperammonemia. Carnitine was not listed in the official nutrition facts table for the tube feeding agents used in this case, and, to the best of our knowledge, there are no reports of tube feeding agents containing carnitine. Given that the “Japanese guidelines for the diagnosis and treatment of carnitine deficiency”8) recommend carnitine supplementation when carnitine deficiency is suspected as a therapeutic diagnosis, we administered 1500 mg/day of L-carnitine according to the instructions accompanying the medication. After 2 weeks of administration, the patient’s GCS rating improved to [E4 V (T) M6] and her blood ammonia decreased to 77 µg/dL. Her state of consciousness returned to the same level as at the time of admission, and we were then able to conduct intensive rehabilitation. During and after the 4-week administration of L-carnitine, no worsening of symptoms—such as convulsive seizures—was observed. As a result, the patient was eventually able to perform gait training using an ankle-foot orthosis and was capable of oral intake of food and liquids. The patient was finally discharged from our rehabilitation center about 3 months after halting L-carnitine administration. Up to this point, symptoms that may have been caused by carnitine deficiency did not recur. The clinical course of Case 1 is shown in Fig. 1.
| WBC | 7200 | /µL | Total cholesterol | 184 | mg/dL | |
| RBC | 3.57 | ×106 /µL | Triglycerides | 345 | mg/dL | |
| Hemoglobin | 11.2 | g/dL | HDL-C | 37 | mg/dL | |
| Platelet | 248 | ×103 /µL | LDL-C | 98 | mg/dL | |
| Albumin | 2.8 | g/dL | ||||
| AST | 17 | U/L | Arterial blood gas analysis | |||
| ALT | 22 | U/L | pH | 7.48 | ||
| Ammonia | 142 | µg/dL | pCO2 | 34.2 | mmHg | |
| Glucose | 84 | mg/dL | pO2 | 89 | mmHg | |
| BUN | 7.8 | mg/dL | HCO3− | 25.1 | mmol/L | |
| Creatinine | 0.61 | mg/dL | Base excess | 2.5 | mmol/L | |
| Na | 138 | mEq/L | Saturated O2 | 97.5 | % | |
| K | 4.5 | mEq/L | ||||
| Cl | 105 | mEq/L | ||||
Not all tests were performed on the same day. Blood gas tests were not performed during a seizure.
WBC, white blood cell; RBC, red blood cell; BUN, blood urea nitrogen; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; pO2, arterial O2 pressure; pCO2, arterial CO2 pressure.
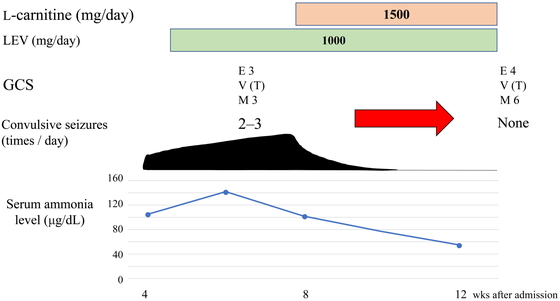
The clinical course of Case 1. LEV, levetiracetam.
A man in his thirties was admitted to an acute care hospital for cerebral hemorrhage caused by a ruptured cerebral arteriovenous malformation. During acute care, aspiration pneumonia, recurrent cerebral hemorrhage, and symptomatic epilepsy were observed, and a tracheostomy was performed. After 5 months of acute treatment, the patient’s general condition had stabilized and his epilepsy was under control. He was then transferred to our rehabilitation center. Physical examination on admission showed the following: height, 170 cm; body weight, 60 kg; body mass index, 20.7; body temperature, 36.8 °C; blood pressure, 120/75 mmHg. In addition, physical examination revealed severe quadriplegia, cognitive dysfunction, aphasia, and severe dysphagia. As a result, the patient required full assistance in his daily activities and was fed through a nasogastric tube. His consciousness was impaired with a GCS rating of [E4 V (T) M6], and he was able to respond to commands with only minor movements such as looking and closing his eyes. After about 2 months of intensive rehabilitation, the patient was able to sit in a wheelchair and was capable of simple communication despite aphasia and attention deficit. However, from around 3 months after transfer to our hospital, he became poorly responsive to our commands. Gradually, tonic seizures (only in the upper limbs), generalized tonic-clonic seizures, and frequent cramps were observed. Disorders of consciousness were also observed, and his GCS rating deteriorated to [E3 V (T) M3]. Recurrence of symptomatic epilepsy was suspected, and the dosage of anticonvulsants and muscle relaxants was increased. The frequency of convulsions decreased from four times a week to about twice a week, the frequency of cramps decreased from five or six times a day to about one or two times a day but remained, and the disorder of consciousness did not improve. AST, ALT, blood glucose, and electrolytes were within normal limits, and urine ketones were negative; however, ammonia was 120 µg/dL. Computed tomography showed no abnormalities in the morphology of his liver. As with Case 1, to the best of our knowledge, carnitine was not a component of the tube feeding agents used in Case 2. Therefore, we suspected carnitine deficiency as a nonhepatic hyperammonemia and examined the patient closely. The free carnitine concentration was as low as 21 µmol/L (normal range is 36–74 μmol/L) and total carnitine was 23.4 µmol/L (normal range is 45–91 μmol/L). Table 2 shows blood test data from this period. After commencing administration of L-carnitine at 1500 mg/day, the symptoms of the patient gradually improved. After 4 weeks of L-carnitine administration, ammonia improved to 77 µg/dL and the GCS rating had improved to [E4 V (T) M6]. At this point, L-carnitine administration was terminated. After the start of L-carnitine administration, no convulsive seizures or cramps were observed. Stabilization of the patient’s general condition and sustained attention allowed active rehabilitation, which contributed greatly to subsequent functional improvement, including the eventual ability to hold a seated position for a short time. Two months after the end of L-carnitine administration, we were able to close his tracheostoma, after which he was also able to take all meals orally. We did not retest the patient after L-carnitine administration, so we do not know whether or how his blood carnitine levels changed, but symptoms that could have been caused by carnitine deficiency did not recur at least until he was discharged from our rehabilitation center about 3 months after the end of L-carnitine administration. The course of Case 2 is shown in Fig. 2.
| WBC | 8100 | /µL | Total cholesterol | 167 | mg/dL | |
| RBC | 4.56 | ×106 /µL | Triglycerides | 88 | mg/dL | |
| Hemoglobin | 14.1 | g/dL | HDL-C | 51 | mg/dL | |
| Platelet | 334 | ×103 /µL | LDL-C | 100 | mg/dL | |
| Albumin | 3.5 | g/dL | Total carnitine | 23.4 | µmol/L | |
| AST | 16 | U/L | Free carnitine | 21.0 | µmol/L | |
| ALT | 26 | U/L | Acyl carnitine | 2.4 | µmol/L | |
| Ammonia | 120 | µg/dL | ||||
| Glucose | 89 | mg/dL | Arterial blood gas analysis | |||
| BUN | 15.9 | mg/dL | pH | 7.41 | ||
| Creatinine | 0.75 | mg/dL | pCO2 | 36 | mmHg | |
| Na | 140 | mEq/L | pO2 | 92 | mmHg | |
| K | 4.3 | mEq/L | HCO3− | 22.1 | mmol/L | |
| Cl | 101 | mEq/L | Base excess | −1.6 | mmol/L | |
| Saturated O2 | 97.3 | % | ||||
Not all tests were performed on the same day. Blood gas tests were not performed during a seizure.
WBC, white blood cell; RBC, red blood cell; BUN, blood urea nitrogen; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; pO2, arterial O2 pressure; pCO2, arterial CO2 pressure.

The clinical course of Case 2. CBZ, carbamazepine; LEV, levetiracetam.
Our two cases indicate the following two points. First, patients with disorders of consciousness after stroke could include those with disorders of consciousness caused by carnitine deficiency. In such cases, administration of L-carnitine might facilitate improvement. Second, measurement of blood ammonia concentration could be useful for diagnosis of carnitine deficiency in patients with post-stroke disorders of consciousness.
Depending on the size and location of the brain injury, patients who have suffered a stroke may present with a variety of symptoms, often including disorders of consciousness. In addition, hydrocephalus and post-stroke epilepsy may also appear during the course of the disease, and disorders of consciousness may appear or worsen. However, knowledge of carnitine deficiency in patients after stroke is limited, and little is known about carnitine deficiency other than that caused by inborn errors of metabolism, epilepsy, cirrhosis, dialysis, or drug-induced deficiency. As a result, medical staff in charge of post-stroke rehabilitation might not be sufficiently informed about carnitine deficiency. For this reason, it may be difficult to achieve a differential diagnosis of carnitine deficiency in patients with disorders of consciousness after stroke. With Case 1, we were fortunate to have an expert point out that the patient might be deficient in carnitine. However, for patients without inborn errors of metabolism (as in Case 1), Japanese medical insurance did not cover the cost of carnitine measurement and rendered the analysis expensive at the time. We so declined to measure blood carnitine levels and could not definitively diagnose carnitine deficiency. However, Japanese guidelines8) state that treatment should proceed when a deficiency is suspected. Therefore, we decided to administer L-carnitine to the patient at 1500 mg/day according to the instructions and observed a favorable outcome. As a therapeutic diagnosis, we believe that the patient in Case 1 most likely had carnitine deficiency. Figure 3 shows a flowchart for the diagnosis of carnitine deficiency, as indicated in the guidelines.8) According to this chart, carnitine deficiency is considered highly likely to occur when free carnitine is between 20 and 36 μmol/L, and L-carnitine supplementation should be considered based on clinical symptoms. The guidelines also point out that carnitine deficiency occurring outside of inborn errors of metabolism is easily missed and may not be noticed until severe symptoms develop. Therefore, the possibility of carnitine deficiency should always be considered when a condition that can cause it is observed. It is believed that 75% of the required amount of carnitine is taken from the diet and the remaining 25% is synthesized in the body.9,10,11) Therefore, long-term use of a carnitine-free diet is thought to increase the risk of carnitine deficiency.3,4) After stroke, some patients are tube fed because of dysphagia, but the number of tube feeding agents that clearly state the carnitine content, or that even contain carnitine, is still few in Japan, although the number has gradually increased in recent years.
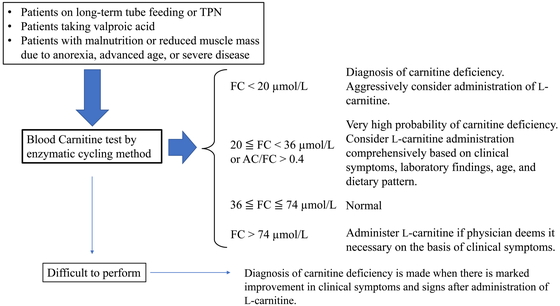
Blood carnitine test and diagnosis and treatment of carnitine deficiency.8) TPN, total parenteral nutrition; FC, free carnitine; AC, acylcarnitine.
In our two cases, the patients were tube fed for more than 6 months with a tube feeding agent that did not specify the carnitine content. This may have led to carnitine deficiency and disorders of consciousness as a symptom of hyperammonemia. If the cause of a patient’s disorders of consciousness is not properly identified, treatable disorders might be overlooked. Especially in the cases of patients who have suffered a stroke, this might deprive them of adequate rehabilitation opportunities at a time when intensive rehabilitation is crucial. To the best of our knowledge, this is the first report of improvement in disorders of consciousness in post-stroke patients treated with carnitine. In patients with post-stroke disorders of consciousness, the possibility of carnitine deficiency should be considered after assessing known risks, such as tube feeding.
Furthermore, measurement of blood ammonia concentration may be useful in diagnosing carnitine deficiency in patients with disorders of consciousness after stroke. Given that carnitine promotes ammonia metabolism via carbamoyl phosphate synthetase 1,12,13) it is known that hyperammonemia can occur as a result of carnitine deficiency.12,13) However, some reports have suggested that carnitine deficiency is unlikely to be associated with hyperammonemia in bipolar patients taking valproic acid,14) and that there is no correlation between free carnitine concentration and ammonia concentration in patients with liver cirrhosis.15) This suggests that the correlation between carnitine deficiency and hyperammonemia differs depending on the cause and background of carnitine deficiency, and that hyperammonemia might not necessarily occur in patients with carnitine deficiency. In our two cases, both patients had hyperammonemia. This led to the administration of L-carnitine for carnitine deficiency, which resulted in improved outcomes. In patients with stroke, measurement of ammonia concentration in the blood might be useful in diagnosing carnitine deficiency. Although we considered carnitine deficiency in the context of hyperammonemia, it is important to note that there are several other causes of nonhepatic hyperammonemia. For example, hyperammonemia is known to occur after generalized tonic-clonic seizures. In such circumstance, ammonia levels return to the normal range after 7–8 h.16) In our case, none of the ammonia levels presented were measured within 8 h after the seizure. Therefore, it is unlikely that the hyperammonemia was caused by a seizure. Drug-induced hyperammonemia could also exist. The antiepileptic drugs valproic acid and carbamazepine have been implicated in both hyperammonemia and carnitine deficiency. In our two cases, the patients had seizures in the acute phase of stroke and were taking levetiracetam as an antiepileptic drug. Levetiracetam is associated with a low risk of hyperammonemia.17) Although valproic acid and carbamazepine, which are associated with a high risk of hyperammonemia, were administered during the course of the patient’s illness, these agents were not the cause of the elevated ammonia because the patient had shown hyperammonemia prior to these medications.
Other metabolic diseases, urinary retention, and constipation may also cause hyperammonemia. We searched for evidence of metabolic disease in the usual blood or urine tests but could find none; nor was there any exacerbation of urinary retention or constipation. A more detailed and specialized examination could have revealed a cause of nonhepatic hyperammonemia other than carnitine deficiency. However, this would have required transfer to another hospital and would have consumed more time. Although therapeutic diagnosis is mentioned in the Japanese guidelines, we believe that in our cases the situation was such that administering medication for carnitine deficiency was reasonable and adequately considered.
We observed seizures, disorders of consciousness, and hyperammonemia in our cases. In contrast, there is a report of severe hyperlipidemia with triglycerides of 3000 mg/dL or higher as a symptom of carnitine deficiency caused by long-term tube feeding.18) In our Case 1, the triglycerides level was 345 mg/dL, but it had not changed 1 month after L-carnitine administration (data not shown). Although we did not observe severe dyslipidemia because of carnitine deficiency in our cases, dyslipidemia should be noted as one of the potential symptoms of carnitine deficiency.
Table 3 lists the classification of carnitine deficiency according to etiology, with consideration of acquired medical conditions and iatrogenic causes.4,5) In our two cases, the patients underwent long-term tube feeding without carnitine. Both were classified as “decreased intake” in Table 3, but because we did not measure their lean body mass or skeletal muscle mass, it is not clear whether they corresponded to “decreased body stores” in Table 3. However, both patients conducted their daily lives without problems before the stroke, maintained their weight relatively well during hospitalization, and showed no significant symptoms of thinness. Therefore, we believe that the main cause of carnitine deficiency in our two cases was long-term tube feeding.
| Acquired medical conditions |
| Decreased intake |
| Long-term total parenteral nutrition |
| Dysphasia |
| Malnutrition |
| Malabsorption |
| Strict vegetarianism |
| Decreased body stores |
| Decreased skeletal muscle mass |
| Decreased biosynthesis |
| Liver cirrhosis |
| Chronic renal disease |
| Decreased loss |
| Renal tubular acidosis |
| Iatrogenic causes |
| Hemodialysis |
| Valproate administration |
Information taken from Pons and De Vivo4) and Scaglia and Longo.5)
In stroke rehabilitation wards, there are many elderly patients and patients with dysphagia. Some of these patients are underfed or have advanced muscle atrophy. It is estimated that 98% of carnitine in the body is present in muscles, such as skeletal and cardiac muscle,19,20) and a decrease in muscle mass may lead to a decrease in body stores of carnitine. Patients with dysphagia are at risk of continued tube feeding. In addition, the antiepileptic drug valproic acid has been reported to be implicated in abnormal carnitine metabolism.21,22,23,24) Valproic acid may be used to treat post-stroke epilepsy, although the incidence of post-stroke epilepsy within 1 year of stroke is estimated to be 2.5%–3.5% in stroke survivors.25,26) Therefore, there is substantial overlap between a patient profile requiring rehabilitation and the classification of carnitine deficiency caused by acquired medical conditions and iatrogenic causes; these would be risk factors for carnitine deficiency. As shown in Fig. 3, Japanese guidelines8) list the following as candidates for carnitine measurement: long-term tube-fed patients, patients on oral valproic acid, malnourished patients, and patients with reduced muscle mass. Therefore, it is possible that there are patients with undiagnosed carnitine deficiency in rehabilitation wards. In our cases, measurement of ammonia led to the diagnosis, and successful diagnosis and treatment of carnitine deficiency facilitated progress in rehabilitation of the patients afterward. Accurate diagnosis and treatment of carnitine deficiency, which may be an inhibitor of rehabilitation, may lead to improvements in physical function and ADL. Therefore, it is important for medical professionals working in rehabilitation wards to consider carnitine deficiency as part of their management of nutrition. If carnitine deficiency is suspected, measurement of carnitine should be considered.
Our two cases provide important insights into carnitine deficiency in patients after stroke, but they also have some limitations. In both cases, we considered the patient to be carnitine deficient and administered L-carnitine, but the dosage was controversial. We administered 1500 mg/day of L-carnitine according to instructions accompanying the medication. The Japanese guidelines8) suggest dosages of 1500 mg/day for hyperammonemia caused by cirrhosis, 750–1000 mg/day for cramps, and 5 mg/kg per day for long-term tube-fed patients. The dosage for long-term tube-fed patients with disorders of consciousness and convulsive seizures, such as in the present cases, is not specified. In considering dosage, side effects and overdose should also be considered. The instructions accompanying the medication list gastrointestinal symptoms such as nausea, vomiting, and diarrhea as side effects of L-carnitine. They indicate a maximum dosage of 3 g/day, and a maximum single dose of 1 g. L-Carnitine is efficiently absorbed in the gastrointestinal tract when taken in small amounts, but when taken in large amounts, the transporter is saturated and bioavailability is only about 10%–20%.8) Although the safety of oral L-carnitine administration is considered high because there is an upper limit to the amount that can be absorbed, clinicians should remain aware of the side effects noted above.
For these cases, it is also unclear what duration of tube feeding was required to cause carnitine deficiency. The degree of carnitine deficiency may differ depending on the duration of tube feeding, and therefore the appropriate dosage may vary depending on the duration of tube feeding. In our experience, not all patients on long-term tube feeding develop symptoms of carnitine deficiency (data not shown). This indicates that unknown factors may be involved. The prevalence of carnitine deficiency in rehabilitation wards, for example, is unknown, and these factors need to be clarified.
To the best of our knowledge, this is the first report in which L-carnitine was administered to a patient with impaired consciousness after stroke with the result that symptoms improved. It is possible that carnitine deficiency is overlooked in some patients in rehabilitation wards, and measurement of ammonia may be useful in its detection. Because carnitine deficiency might interfere with active rehabilitation, nutritional management with attention to carnitine deficiency is important in rehabilitation wards.
The authors declare no conflict of interest.