2023 Volume 8 Article ID: 20230025
2023 Volume 8 Article ID: 20230025
Objectives: This study explored the relationship between clinical severity of ulnar neuropathy at the elbow (UNE) and ulnar nerve cross-sectional area (CSA) by ultrasound examination to identify appropriate measurement sites for UNE diagnosis and evaluation.
Methods: In this retrospective analysis, we examined the arms of 37 patients diagnosed with UNE and those of 34 individuals as controls. The ulnar nerve CSAs were measured at 2 cm distal to the tip of the medial epicondyle (dME), the tip of the medial epicondyle (ME), 2 cm proximal to the tip of the medial epicondyle (pME), and any site showing the maximum CSA between the dME and pME (largest dpME). The modified McGowan classification (grades I, IIA, IIB, and III) was used to rate the clinical severity of UNE.
Results: For all sites, the CSAs were significantly correlated with clinical severity. The sites showing the maximum CSA were inconsistent between controls and grade IIA patients. Grade IIB patients showed the largest CSA at the ME in the majority of patients. In grade III patients, maximum CSA occurred only at the ME.
Conclusions: Serial assessment to detect nerve enlargement at multiple sites was beneficial for mild UNE patients with weakness of the ulnar distal muscles with Medical Research Council (MRC) score of 4 or higher (grade IIA). For severe UNE patients with weakness of the ulnar distal muscles classified as MRC3 or less (grades IIB, III), the most efficient method for detecting enlarged nerves was to initially measure the CSA at the ME.
Ulnar neuropathy at the elbow (UNE) is the second-most common entrapment neuropathy, with physical examinations and electrodiagnostic techniques being commonly used for evaluation and diagnosis.1,2,3,4,5,6,7,8,9,10) However, electrodiagnostic examination is rather invasive and often has limitations in terms of detecting the site of lesions in patients with axonal loss.5,8,9,10)
Because ultrasonographic examination is less expensive, less invasive, and less time-consuming than magnetic resonance imaging (MRI) and electrodiagnostic study, it has recently been used for the diagnosis of entrapment neuropathies, including UNE.1,2,3,10) The ulnar nerve cross-sectional area (CSA) commonly increases around the elbow in patients with UNE.4) Although evidence-based guidelines have not yet been established, a recent review suggested that ultrasonographic measurement of the nerve diameter or CSA should be performed to diagnose and localize the lesion site in patients with symptoms suggestive of UNE.5) In addition, clinical attention has been given to the significance of ultrasonographic examination in detecting UNE with extremely mild clinical severity to be diagnosed using electrodiagnostic methods.6)
As clinicians performing ultrasound examinations on patients with UNE, we have frequently noted that CSA enlargement of the ulnar nerve at the elbow is prominent in patients with severe weakness (Medical Research Council [MRC] score 3 or less) of intrinsic muscles innervated by the ulnar nerve. However, although some studies6,7,8,9) have examined the relationship between ulnar nerve CSA and clinical severity based on physical findings, the results are inconsistent.
Moreover, although some studies have failed to demonstrate a significant correlation,7,8) Pelosi and Mulroy6) identified a positive relationship between the maximum CSA around the elbow and clinical severity. However, the description of the appropriate site for CSA measurement was unclear. Omejec and Podnar9) also reported that the maximum CSA around the elbow of clinically severe UNE with marked muscle weakness and atrophy was significantly larger than that of clinically milder UNE.
These inconsistent results may be caused by heterogeneity in patient severity across studies, different use of clinical severity classification, and/or variability of parameters analyzed, including measurement sites. We believe that a more detailed classification of severity based on muscle strength as an index for clinical classification is important, and CSA changes with clinical severity should also be examined by measuring CSA at multiple sites.
Therefore, this study aimed to explore the relationship between clinical severity and CSA and to identify appropriate measurement sites for the diagnosis and evaluation of UNE. To grade the clinical severity of UNE, we used the modified McGowan classification of Goldberg et al.,11) which adopts a grading classification bordered by MRC3 and analyzed four CSA measurements around the elbow.
This retrospective, cross-sectional, and observational study was approved by the Institutional Review Board of Tokai University School of Medicine (No. 12R232; January 11, 2013). Because of the retrospective nature of the study, the requirement of informed consent from the patients was waived.
ParticipantsUNE Group (Patients with UNE)The affected arms (82 in total) of 70 consecutive patients were examined based on their symptoms and signs that were suggestive of UNE. All patients had been referred to the Department of Rehabilitation Medicine at a university hospital for electromyography between January 2008 and February 2011. These patients included 50 men and 20 women aged 17–85 years, with a median age of 57.5 years. Four arms that had previously undergone elbow surgery were excluded.
Figure 1 shows a flowchart of patient selection, including the inclusion and exclusion criteria for the study. The diagnostic process for UNE in the arms was based on the method described by Beekman et al.10) A diagnosis of UNE was made solely based on clinical grounds when sensory disturbance of both the dorsal and palmar sides of the lateral side of the fourth finger and the whole of the fifth finger (with involvement of the dorsal cutaneous branch) as well as weakness of the ulnar distal muscles (abductor digiti minimi [ADM] and the first dorsal interosseous [FDI]) or ulnar proximal muscles (flexor digitorum profundus [4th and 5th] and flexor carpi ulnaris) were found. Twenty arms were subsequently diagnosed with UNE and were suitable for inclusion into this study.
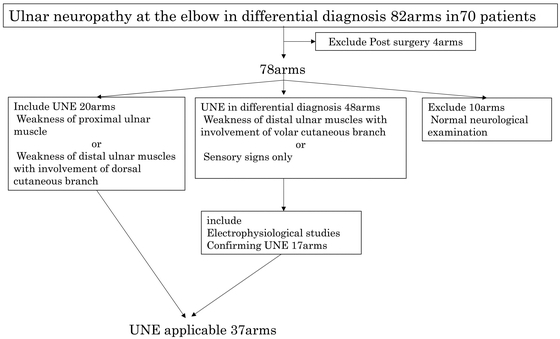
Flowchart of participant selection process, including the inclusion and exclusion criteria.
Overall, 48 arms showed clinical signs suggestive of UNE but did not satisfy the above diagnostic criteria (weakness of distal ulnar muscles with sensory involvement of the volar cutaneous branch or sensory signs only). In this group of patients, UNE was diagnosed in conjunction with electrodiagnostic results. In accordance with the American Academy of Neurology summary statement (1999),12) we diagnosed the condition as electrophysiological UNE when the following occurred: (1) differential slowing (10 m/s across the elbow) and/or (2) conduction block across the elbow (more than 20% decrease in the amplitude of compound action potential [CMAP] across the elbow), in addition to absolute motor conduction velocity of less than 50 m/s from above the elbow to below the elbow. Therefore, the condition for 17 arms was diagnosed as UNE, and these patients were included further in this study. The remaining 31 arms included 3 arms with non-localizing ulnar neuropathy, 9 arms that were electrophysiologically within the normal limit, 10 arms with polyneuropathy, 5 arms with mononeuritis multiplex, and 4 arms with radiculopathy.
In the remaining 10 arms, patients only had symptoms of sensory disturbance, but no abnormal findings that were suggestive of UNE upon neurological and electrodiagnostic examinations (negative findings, polyneuropathy, Guyon tunnel syndrome, and carpal tunnel syndrome for 6, 2, 1, and 1 arms, respectively). Therefore, none of these 10 arms were included in the study.
Consequently, a total of 37 arms from 33 patients (28 men and 5 women; aged 27–85 years; median age, 60 years) were examined (definitively diagnosed as having UNE): 7 arms with osteoarthritis of the elbow (OA), 2 arms with extroversion of the elbow (caused by childhood elbow fractures), 1 arm with rheumatoid arthritis (RA), and the remaining 27 arms with idiopathic UNE. None of the patients had comorbidities, such as diabetes mellitus, hypothyroidism, or connective tissue disease.
All the 33 patients were right-handed, and the affected side was the right side in 19 arms, the left side in 6, and both sides in 6 patients (12 arms). The time lag between the first complaint and ultrasonographic examination varied considerably, from 2 days to 35 years, with a median of 2 months. We used the modified McGowan classification as described by Goldberg et al. (Table 1)11) to rate the clinical severity of UNE, as shown in Table 2.
| Grade | Clinical findings |
| I | Only symptoms without abnormal objective findings |
| IIA | Sensitivity loss and good intrinsic strength (MRC grade 4) without muscle atrophy |
| IIB | Sensitivity loss and fair intrinsic strength (MRC grade 3) with intrinsic atrophy |
| III | Severe sensitivity loss, severe motor weakness (MRC grade 2–0), and marked atrophy |
As described by Goldberg et al.11)
| Control group | Patient subgroups | P | |||
| IIA | IIB | III | |||
| Arms (n) | 34 | 12 | 16 | 9 | |
| Age (years) | 59.5/32–85 | 60/27–81 | 60/28–85 | 59.5/32–85 | 0.225 |
| Male/female (n) | 26/8 | 9/3 | 14/2 | 8/1 | 0.689 |
| Time from first symptoms (months) | - | 2.5/1–6 | 2/1–420 | 3/0.25–96 | 0.804 |
Data displayed as number or median/range.
The control group included 34 arms of 22 individuals (18 men and 4 women; aged 32–86 years; median age, 46 years). One participant was left-handed, whereas the rest of the 21 participants were right-handed. Moreover, CSA data from 24 arms were obtained from 12 healthy hospital employees. The data for the remaining 10 CSAs were obtained from non-affected arms of patients with trauma to the contralateral elbow or wrist joint were also included. None of the patients had any symptoms or history of neurological disorders. No significant differences were found in age (Mann–Whitney U test, P=0.094) or sex proportion between the control and UNE groups (χ-square test, P=0.439).
Ultrasonographic ExaminationThe ulnar nerves in the UNE group (patients with UNE) and control group (arms of healthy patients without UNE and arms of healthy participants) were examined. Ultrasonographic studies were performed using an ultrasonographic device (Aplio XV, Canon Medical Systems, Tochigi Japan; and LogiQ7, GE Healthcare Japan, Tokyo Japan) with a 12-MHz linear array transducer.
Participants were placed in the supine position with their arms bent at 15° to facilitate imaging around the elbow, and several operators with experience in neuromuscular ultrasound conducted the examinations. To obtain accurate data on CSA, we ensured that the transducer was placed perpendicular to the nerve, which was then immediately traced circumferentially within the hyperechoic rim to an accuracy of 1 mm2 (Fig. 2a,b).

Examples of ultrasound images of the ulnar nerve (arrow) in the axial view. (a) A healthy participant (examined at the ME) with CSA=7 mm2. (b) A patient with UNE (examined at the ME) with CSA=22 mm2. The area surrounded by the dotted line is the ulnar nerve cross-sectional area.
Shook et al.5) recommended obtaining the CSA at the level of the medial epicondyle and across the elbow segment from at least 2 cm proximal to 2 cm distal to the site. Therefore, the ulnar nerve CSAs were measured at the following four sites: (1) 2 cm distal to the tip of the medial epicondyle (dME), (2) the tip of the medial epicondyle (ME), (3) 2 cm proximal to the tip of the medial epicondyle (pME), and (4) any site showing the maximum CSA between the dME and pME (the largest dpME).
Statistical AnalysisStatistical analyses were performed using IBM SPSS Statistics for Windows version 28 (IBM, Armonk, NY, USA). The distributions of all variables were examined for normality using the Shapiro-Wilk test. When the data were normally distributed, unpaired t-test was used to detect differences in CSA between the control and UNE groups. Moreover, a paired t-test was used to detect differences in CSA between the dominant and non-dominant arms. Furthermore, analysis of variance was used to compare CSA between the control and UNE groups as well as UNE subgroups according to modified McGowan classification, as described by Goldberg et al.11) When the data were not normally distributed, the Mann–Whitney U test was used to detect differences in CSA between the control and UNE groups. Furthermore, the Wilcoxon signed-rank test was used to detect differences in CSA between the dominant and non-dominant arms. Moreover, the Kruskal–Wallis test was used for comparison of CSA between the control and UNE groups as well as UNE subgroups according to the modified McGowan classification.11) We compared every pair of data points using Bonferroni’s method and examined the relationship between CSA and clinical severity using Spearman’s rank correlation. The χ-square test was used to examine the proportion of categorical data.
We obtained ultrasonographic images of all ulnar nerves in the control and UNE groups (Fig. 2). The CSAs were not normally distributed in both groups (Shapiro-Wilk test, P<0.05); therefore, we used non-parametric methods for the statistical analyses.
CSA for the Control GroupThe CSA data were obtained for both sides of the 12 healthy participants. No significant right–left difference was noted in the CSAs for any of the four sites (Wilcoxon signed-rank test, P=0.587, 0.237, 0.339, and 0.284 for dME, ME, pME, and the largest dpME, respectively). Therefore, the data for both sides were treated equally and analyzed as reference data. The 10 CSAs obtained from non-affected arms of patients with trauma to the contralateral elbow or wrist joint were also included. Sex differences in the CSAs were not observed for any site (Mann–Whitney U test, P=0.432, 0.705, 0.618, and 0.485 for dME, ME, pME, and the largest dpME, respectively).
In the control group, the median CSAs at the four measurement sites were as follows: dME, 6.5 mm2 (range, 4–9 mm2); ME, 7 mm2 (range, 4–10 mm2); pME; 7 mm2 (range, 4–9 mm2); largest dpME, 8 mm2 (range, 6–10 mm2). The value of the largest dpME was significantly larger than that of the other three sites (dME, ME, and pME) (Kruskal–Wallis test, P<0.05; Bonferroni adjustment, P<0.05), whereas no significant difference was noted in comparison of the other three sites (dME, ME, and pME).
Severity Classification of UNENone of the arms belonged to the grade I subgroup, with no significant difference noted in age between the control and the three UNE subgroups (Kruskal–Wallis test, P=0.225). Furthermore, no significant difference was noted in sex proportions between these groups (χ-square test, P=0.689). The time from the first symptoms varied considerably among the UNE subgroups; however, no significant difference was noted in these values (Kruskal–Wallis test, P=0.804).
The causative diseases varied significantly among the patient subgroups (χ-square test, P<0.05). All 12 UNE patients in the grade IIA subgroup and 13 of the 16 UNE patients in the grade IIB subgroup were idiopathic, and each of the remaining 3 arms of patients in the grade IIB subgroup had OA, extroversion of the elbow, or RA. In the grade III subgroup, UNE for 2 arms was attributed to idiopathic causes and UNE for 6 arms was attributed to OA. UNE related to OA and idiopathic UNE were predominantly found in more severe and milder UNE cases, respectively.
Comparison of CSA between Control Group and UNE GroupsThe CSAs of UNE patients were larger than those of the control participants for all measurement sites (Mann–Whitney U test, P<0.05 for all comparisons). For the UNE group, the median CSAs were as follows: dME, 10 mm2 (range, 5–20 mm2); ME, 14 mm2 (range, 5–49 mm2); pME, 9 mm2 (range, 4–22 mm2); largest dpME, 14 mm2 (range, 5–49 mm2). The CSAs at the ME and the largest dpME (with no statistically significant difference in these two values) were larger than those of the other two sites (with no statistically significant differences in these two values) (Kruskal–Wallis test, P<0.05 among the four sites, adjusted by the Bonferroni method).
Comparison of CSA between Control Group and Three Subgroups of Patients with UNEFigure 3 compares the CSAs determined at each site across the UNE subtypes. At all four sites, the CSAs of the grade IIB and III subgroups were larger than those of the control group and the grade IIA subgroup. However, no statistically significant difference was noted between the grade IIA and IIB subgroups at pME (Kruskal–Wallis test, P<0.05; Bonferroni adjustment, P<0.05).
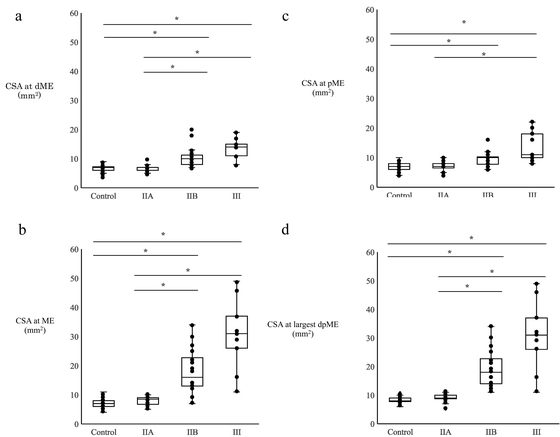
CSAs of the control group and the UNE subgroups measured at (a) dME, (b) ME, (c) pME, and (d) the largest dpME. A significant difference is noted in the CSA among the four groups (Kruskal–Wallis test, P<0.05). Asterisks indicate significant difference in every pair of controls and each patient subgroup (Bonferroni method, P<0.05). In each group, multiple arms with the same CSA are represented by the same data point.
For all four sites, the CSAs showed no significant difference between the grade IIB and III subgroups (Bonferroni adjustment, P=1.00, 1.00, 0.84, and 1.00 at dME, ME, pME, and the largest dpME, respectively) and between the control group and grade IIA subgroup (Bonferroni adjustment, P=1.00, 1.00, 1.00, and 1.00, at dME, ME, pME, and the largest dpME, respectively). Furthermore, the CSAs were significantly correlated with clinical severity for all sites (Spearman’s rank correlation, rs=0.743, 0.798, 0.632, and 0.831; all P<0.05 at dME, ME, pME, and the largest dpME, respectively).
Location of Largest dpMEThe distribution of the sites for the largest CSA is shown in Fig. 4. In the control group, the maximum CSA was observed at any one or two sites among dME, ME, and pME. In the grade IIA subgroup, the maximum CSA was also observed at one or two sites among dME, ME, and pME, with sites of the maximum CSA in this subgroup being distributed in a pattern similar to that noted in the control group. Unlike the control group, this subgroup included one arm that showed the maximum CSA between dME and ME. In the grade IIB subgroup, the maximum CSA was observed at one site in all arms. The common site was the ME, while one arm showed the maximum CSA at pME and one arm showed between dME and ME. In the grade III subgroup, the maximum CSA was observed at ME in all arms. No participant simultaneously showed the maximum CSA at all three sites of dME, ME, and pME.

Distribution of the largest CSA site for the UNE subgroups. The vertical axis shows percentages, and the horizontal axis shows the control group and the UNE subgroups. The numerical values in the bar graph indicate the percentages (%) of sites of the maximum CSA in the control group and each severity grade subgroup. The site of the maximum CSA is indicated by shading (key provided on figure).
We observed a clear trend toward a greater CSA for severe UNE. These findings support the results of previous reports6,9,13,14) that ulnar nerve enlargement around the elbow on ultrasound examination increases with clinical severity. In related work, Okamoto et al.13) investigated the diameter of the major axis of the nerve. In the present study, we used CSA instead of diameter because nerves are not always circular or elliptical.15) Moreover, the probe was always perpendicular to the running direction of the nerve so as to obtain an accurate horizontal section.
Previously, Mondelli et al.7) investigated the correlation between CSA and severity grade based on physical findings; however, they did not demonstrate a significant correlation between the severity grade and CSA around ME. In their study, clinical severity classification was based on the authors’ original four-point method7): stage I (only subjective symptoms), stage II (objective sensory loss), stage III (sensory and motor deficits), and stage IV (severe sensory loss, no voluntary activity, and muscle atrophy). However, 90% of the patients had mild neuropathy with only sensory disturbance (stages I and II). Furthermore, Ellegaard et al.8) reported no positive relationship between clinical severity and the CSA. They used the original McGowan classification16) for staging clinical severity (grade I, minimal lesions with no detectable motor weakness of the hand; grade II, intermediate lesions; grade III, severe lesions with paralysis of one or more of the ulnar intrinsic muscles). For all 41 arms investigated, 15, 21, and 5 arms were classified as grade I, II, and III UNE, respectively. The grading index of motor impairment adopted in the original McGowan classification was rudimentary. These methodological issues may interfere with obtaining a significant relationship between CSA and the clinical severity of UNE.
It this study, no significant difference was noted between the control group and the grade IIA subgroup. However, caution should be used before concluding that nerve enlargement does not occur in mild cases. Omejec and Podnar17) found the maximal CSA 1 cm distal to the ME, which is a site that we did not use in the present study. In addition, Mamarabadi et al.18) reported clinical significance in the comparison of right–left CSA to enhance the detection of nerve swelling for UNE patients. Considering these findings, future studies may need to re-investigate the nerve swelling in mild cases in detail.
The majority of arms with OA or extroversion of the elbow were in the grade IIB and III subgroups. Therefore, the CSA was larger for these diseases. Because of the small number of cases in the present study, the effect of diseases on CSA could not be rigorously analyzed. Meanwhile, Okamoto et al.13) did not show any clear tendency for the diameter of the major axis to increase with OA, regardless of disease severity. In contrast, the diameter of the major axis appeared to be greater in the extroversion of the elbow than in the other diseases. However, further studies are necessary to investigate the relationship between nerve size, causative disease, and clinical severity of UNE.
Site of CSA Measurement for Evaluating UNEThe use of different observation sites for evaluating UNE does not yield consistent findings among investigators. For example, according to a meta-analysis by Chang et al.,4) many studies examined the areas between 4 cm distal and 4 cm proximal to the ME. Mondelli et al.7) investigated the areas 2 cm distal and 2 cm proximal to the ME, which is different from that investigated by Chang et al.4) This difference is an example of the variation of the investigation site among investigators.
Although many reports cite ME as the measuring site because ultrasonographic abnormalities are easily detected,4) the increase in CSA has sometimes been noticeable in other areas.17,19,20) This may be caused by differences in the severity of the disease or by the diverse pathophysiology of UNEs.21,22) Moreover, the site of the main lesion is modified when OA, extroversion of the elbow, or repetitive dislocation is added to the lesion.22) Therefore, ultrasonography should be performed at multiple locations. In the present study, an increase in CSA was noted with UNE severity, irrespective of the recording site.
Several studies have previously analyzed the maximum CSA value.6,12,17,20,23) Therefore, we also identified the maximal CSA between the dME and pME. In the control group and the grade IIA subgroup, the maximum CSA was observed not only at the ME but also at other sites. In the grade IIB subgroup, the proportion of arms showing the maximum CSA at ME was increased. In the grade III subgroup, which included the most severe UNE cases, the maximum CSA was observed at ME in all arms. As shown in Fig. 3a–d, the CSA at sites other than ME (i.e., dME and pME) was larger in some arms in the grade IIB and III subgroups than in the arms in the control group. Swelling was observed at multiple sites.
In lesions at the humeroulnar aponeurotic arcade, proximal swelling from the site of strangulation distal to the ME has been noted.4,17) When the lesion reached the ME as the severity increased, repeated physical stimulation at the site of the fulcrum of elbow flexion movement may have amplified the increase in CSA. It has also been reported that retrocondylar groove compression can cause nerve damage both proximally and distally to the compression site,17) and mechanical stimulation at the ME site can have similar effects. These mechanisms may explain why the location of the maximum CSA in severe UNE cases is concentrated in the ME region.
Considering the above findings, the most efficient method for detecting nerve swelling is to measure the CSA first at the ME for severe cases with ulnar distal muscles classified as MRC3 or less. However, a considerable number of mild cases showed a maximum CSA proximal and distal from the ME. Ultrasonographic examination, which allows serial scanning along the courses of nerves, facilitates the detection of changes in the cross sections of nerves and can depict changes at multiple sites. Therefore, serial scanning of multiple sites, including areas 1 cm proximal and 1 cm distal to the ME, is important to accurately detect nerve enlargement in mild cases.
LimitationsThis study has some limitations. First, the number of cases included was small in both the control and UNE groups/subgroups. Second, the sensitivity and specificity of diagnostic accuracy were not examined. Third, there was bias in the distribution of severity in the UNE group, because no patients were classified in the grade I subgroup according to the modified McGowan classification.11) Fourth, ultrasonographic findings were not analyzed together with electrodiagnostic measurements. In the future, it will be critical to consider the relationship between the severity of the physical findings and the site of CSA measurement, to study their sensitivity and specificity, and to study their relationship with the pathophysiology of neuropathy obtained by electrodiagnosis. Such advances will allow CSAs to be used for UNE diagnosis in actual clinical practice.
The CSAs around the elbow were significantly correlated with UNE severity, as graded by physical findings. The CSA in the mild UNE subgroup was not significantly different from that in the control group; however, the CSA in the severe UNE subgroup tended to be larger. Although the sites of the maximum CSA in the control and mild to moderate UNE subgroups were inconsistent, those in most severe UNE cases were found only at the ME.
Serial assessment to precisely detect nerve enlargement at multiple sites was beneficial for mild UNE patients with ulnar distal muscles (ADM and FDI) classified as greater than MRC4 or higher (grade IIA). For severe UNE patients with weakness of the ulnar distal muscles (ADM and FDI) classified as MRC3 or less (grades IIB, III), the most efficient method for detecting enlarged nerves was to initially measure the CSA at the ME.
The authors thank the sonographers, Noboru Takanashi MT, Yui Ishii MT, and Yuki Kariya MT, from the Clinical Laboratory Department of Tokai University Hachioji Hospital. The authors also thank the Honyaku Center for English language editing of a draft of this manuscript.
The authors declare no competing interest.