ABSTRACT
Objectives: One of the causes of death in patients with multiple
system atrophy (MSA) is aspiration pneumonia caused by cough dysfunction. This
study aimed to identify an effective approach to improve coughing and to explore
the establishment of criteria for the use of gastrostomy based on cough and
respiratory dysfunctions.
Methods: Eighteen probable MSA patients participated in the study.
They were categorized into air stacking and non-air stacking groups. First, we
investigated how the inspiration volume changes by applying maximum insufflation
capacity (MIC). Second, peak cough flow (PCF) was measured by different cough
augmentation methods: 1) spontaneous coughing (SpC); 2) SpC with MIC
(SpC + MIC); 3) SpC with manually assisted cough (MAC) (SpC + MAC); and 4) SpC
with MIC and MAC (SpC + MIC + MAC). Among these four conditions, PCF values were
compared to determine the most effective approach for cough augmentation.
Receiver operating characteristic analysis was performed on percent forced vital
capacity (%FVC) to determine an index for discriminating PCF below160 L/min,
which indicates a high risk of suffocation, involving SpC and SpC + MIC.
Results: Inspiration volume increased significantly with MIC in both
groups (P < 0.05), and PCF increased significantly with MIC in the air
stacking group (P < 0.01). PCF could not be maintained at 160 L/min when %FVC
fell below 59%, even when MIC was applied.
Conclusions: PCF increases with MIC in patients with MSA. It may be
meaningful to consider the timing of gastrostomy introduction based on the
severity of cough and respiratory dysfunction.
INTRODUCTION
Multiple system atrophy (MSA) is a rare and progressive neurodegenerative
disease.1,2) It was previously classified as olivopontocerebellar
atrophy, Shy-Drager syndrome, or striatonigral degeneration.2,3) According to natural
history studies of the patients with MSA, the average life expectancy from the onset
of motor symptoms is 9.8 years, and the mean age of onset is 60 years.4,5) Aspiration pneumonia is
one of the causes of death in patients with MSA.6) Generally, aspiration pneumonia is caused by a
decrease in cough function.7) Previous studies revealed the effectiveness of
rehabilitative interventions aimed at strengthening the cough function in the
presence of neuromuscular diseases such as Duchenne muscular dystrophy and
amyotrophic lateral sclerosis.8,9) Some studies on respiratory function in MSA have
concluded that respiratory function is maintained, whereas others found that it
gradually deteriorates.10,11) Importantly, vocal cord dysfunction is associated
with life-threatening events such as upper airway obstruction, nocturnal sleep apnea
syndrome, and stridor. In addition, vocal cord dysfunction can lead to the
impairment of coughing.12) However, there has been no report of efforts
seeking effective cough augmentation maneuver in the presence of MSA. In addition,
gastrostomy has also been performed in clinical settings for MSA patients with
inadequate coughing.1,6) After a search of the literature, it appears that no
study has examined the timing of gastrostomy based on cough and respiratory
functions in MSA. Clarification of the timing would promote the planning of
therapeutic interventions and collaborative decision making on treatment. Therefore,
the aims of this study were to: 1) identify the most effective approach to improve
cough function, and 2) explore conditions requiring therapeutic intervention, such
as gastrostomy, based on cough and respiratory functions.
MATERIALS AND METHODS
Ethical Considerations
This study was approved by the Medical Ethics Committee of the International
University of Health and Welfare School of Medicine (Approval number: 21-Im-006)
and was conducted in accordance with the principles of the Declaration of
Helsinki. We registered our study with the University Hospital Medical
Information Network (UMIN) Center before starting examination (UMIN-CTR ID:
UMIN000045378). All procedures were conducted after subjects provided written
informed consent.
Participants
We conducted a single-facility and hospitalized care study. This study was a
non-randomized, prospective intervention study (a cross-sectional study) using
data from the International University of Health and Welfare Ichikawa Hospital
Intractable Disease Center from 1 July 2021 through to 31 May 2023. Study
participants were recruited from patients admitted to the Department of
Neurology of our hospital. In addition, we asked the MSA registry, which
collects information on MSA patients from medical institutions, to introduce
this study and recruit patients.
The study used the following inclusion criteria: 1) clinical diagnosis of
probable MSA based on the second consensus diagnostic criteria13); 2) no
cardiopulmonary diseases such as chronic obstructive pulmonary disease,
myocardial infarction, cardiac insufficiency or similar conditions; 3) no
malignant disease; 4) no neurological or neuromuscular diseases other than MSA;
5) no acute or chronic inflammatory or infectious diseases; 6) no tracheostomy
and/or artificial ventilation before the study; 6) capable of verbal
communication; and 7) no cognitive impairment as confirmed by a Mini-Mental
State Examination (MMSE) score below 21 and a Frontal Assessment Battery (FAB)
score less than 13).
Outcome Measures
Respiratory function, including vital capacity (VC), percent vital capacity
(%VC), and percent forced vital capacity (%FVC), was assessed by spirometry
(Autospiro AS-407; Minato Medical Science, Japan). Respiratory muscle strength
such as percent maximal inspiratory mouth pressure (%PImax) and percent maximal
expiratory mouth pressure (%PEmax) were assessed using a respiratory muscle
strength-measuring device (IOP-01; Kobata Gauge, Japan) by oral
manometry.14) These measurements were recorded while the
patient wore a nose plug and the mouthpiece was firmly held in place by hand to
prevent air leaks. Both respiratory function assessment and respiratory muscle
strength testing were conducted after the patient was allowed to practice the
measurement once. All data obtained in percent predicted values were adjusted
for height, weight, age, and sex by the assessment device.
Forced insufflation using a resuscitation bag (single-use resuscitation bag
adult; Philips, The Netherlands) is an assistance maneuver that can be used to
achieve maximum insufflation capacity (hereinafter, this technique is referred
to as MIC).15) A
pressure gauge (disposable manometer; MPI, Japan) and a simple spirometer
(Haloscale standard respirometer; nSpire Health, UK) were attached to the
resuscitation bag to measure the pressure and expiratory volume,
respectively.16) The participants used a face mask (cough assist
face mask; Philips) to cover the nose and mouth to prevent air leaks. In
addition, an LIC Trainer (Carter Technologies, Japan) with a one-way valve was
used to prevent any expiration leak during forced insufflation with a
resuscitation bag.17) To perform MIC, this study used three forced
air pressures: 20, 30, and 40 cmH2O, and the expiratory volume was
measured. A manually assisted cough (MAC) is a technique in which a hand is
placed on the upper or lower rib cage and pushed inward at the same time as the
patient coughs.18)
This technique assists expiration by pushing the ribcage from the outside.
Inspiratory volume measured without any respiratory maneuver refers to VC.
Cough function was measured as peak cough flow (PCF) by connecting a face mask to
a peak flow meter (Mini-Wright™ Standard Peak Flow Meter; Clement Clarke
International, UK).15) Previous research has established ways to
strengthen cough function in patients with neuromuscular diseases by using
inhalation assistance, exhalation assistance, or both.15) Therefore, in this study, we
applied MIC as inhalation assistance and MAC as exhalation assistance. PCF was
measured by employing four different cough augmentation methods: 1) spontaneous
coughing (coughing without any assistance) (SpC); 2) SpC with MIC (SpC + MIC);
3) SpC with MAC (SpC + MAC); and 4) SpC with MIC and MAC
(SpC + MIC + MAC).8,15) For each condition, PCF was measured three
times and the maximum value was used. The order of measurement for each pattern
was randomly selected.
The Unified Multiple System Atrophy Rating Scale (UMSARS) parts 1, 2, 4, and
Hoehn & Yahr scale (H&Y) were used to assess the severity of disease for
all participants. UMSARS part I assesses impairments affecting activities of
daily living (12 items), and UMSARS part 2 assesses impairments affecting motor
function (14 items). UMSARS part 4 is the global disability score. Each item is
scored from 0 (unaffected/normal) to 4 (severe impairment).19) H&Y is a
parkinsonian motor impairment severity scale from stage 1 (unilateral symptoms)
to stage 5 (wheelchair bound or bed ridden).20) In addition, the Movement Disorder Society
Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3 was used to assess
the severity of motor function. It has 18 questions (33 scoring items). Each
item scores from 0 (normal) to 4 (severe) and total scores are obtained from the
sum of each item score.21) The Scale for the Assessment and Rating of
Ataxia (SARA) was used to assess ataxia. The total score can range from 0
(without ataxia) to 40 (most severe) based on eight assessment points: gait (0–8
points); stance (0–6 points); sitting (0–4 points); speech disturbance (0–6
points); finger chase (0–4 points); nose–finger test (0–4 points); fast
alternating hand movement (0–4 points); and heel–shin slide (0–4
points).22)
Vocal cord movement can be impaired in MSA patients. When the vocal cords do not
open or close completely and become fixed in the midline position, it is a
contributing factor to stridor and nocturnal sleep apnea syndrome, which are
potentially life-threatening conditions.10,12) However, coughing requires the ability to
close the vocal cords and hold the breath.23) Vocal cord dysfunction in MSA patients
means that some may be unable to hold their breath, leading to decreased cough
strength. Therefore, in this study, participants were divided into two groups
based on their ability to hold their breath: the air stacking group and the
non-air stacking group.8) Patients in the air stacking group were able to
hold their breath without leakage during measurement of maximum insufflation
capacity, whereas patients in the non-air stacking group could not fully hold
their breath at the lowest pressure for MIC of 20 cmH2O. For cough
function evaluation, two critical values of PCF (270 and 160 L/min) are used as
criteria for assessing cough strength. When PCF is less than 270 L/min, it
becomes difficult for an individual to produce sufficient sputum, and other
methods of sputum excretion are required.15,24) When PCF falls below 160 L/min, sputum
drainage becomes impossible and suction or endotracheal intubation is
required.24,25,26) These two points are important when assessing
cough function and performing interventions aimed at improving cough
strength.
Statistical Analysis
Statistical analysis software (SPSS statistics version 27; IBM, Armonk, NY, USA)
was used to analyze all data. Data are expressed as mean (± SD) for parametric
variables and ordinal data. The numbers and percentages are reported for nominal
data and the ratio scale. The paired t-test was performed for
two data obtained by changing the conditions for a matched sample. The
two-sample t-test was used to compare parametric variables
between the air stacking and non-air stacking groups. Fisher’s exact test was
used to analyze categorical data. The differences among the four cough
assistance methods were analyzed by repeated measures analysis of variance
(ANOVA) and compared by multiple comparison. Cut-off values (CVs) of PCF below
270 L/min or below 160 L/min at SpC and SpC + MIC in %FVC were examined using
the receiver operating characteristic (ROC) curve. The area under the curve
(AUC), Sensitivity (Sen.), and Specificity (Spe.) were calculated. The Youden
index (Sensitivity + Specificity − 1) was calculated at all points of the ROC
curve to determine the potential CV.
RESULTS
This study included 24 consecutive patients with probable MSA. Of the 24 patients, 6
did not meet the inclusion criteria. Of the 6 patients, 3 were on a tracheostomy
ventilator, 2 showed cognitive decline, and 1 refused to participate in the study.
Therefore, this study examined 18 consecutive patients with MSA.
The demographic backgrounds of the patients are shown in Table 1. Of the 18 patients, 12 were able to hold their
breath (air stacking group). The other 6 patients were unable to hold their breath
(non-air stacking group). Regarding the basic information and the cognitive
function, no significant difference was observed between the groups. However, %VC,
%FVC, %PImax, and %PEmax were significantly higher in the air stacking group than in
the non-air stacking group. Scores for H&Y, SARA, MDS-UPDRS part 3, and UMSARS
part 1, part 2, and part 4 were significantly higher in the non-air stacking group
than in the air stacking group.
Table 1. Participant information
|
All |
Air stacking group |
Non-air stacking group |
P value |
| Basic information |
| n |
18 |
12 |
6 |
- |
| Sex (F/M) |
8/10 |
5/7 |
3/3 |
n.s. |
| Height (cm) |
164.33 (±9.33) |
165.58 (±9.89) |
161.83 (±8.33) |
n.s. |
| Weight (kg) |
58.92 (±16.63) |
60.32 (±16.57) |
56.13 (±17.96) |
n.s. |
| BMI (kg/m2) |
21.70 (±5.09) |
21.78 (±4.43) |
21.55 (±6.69) |
n.s. |
| Age at onset (years) |
56.45 (±8.33) |
56.00 (±9.99) |
57.33 (±3.88) |
n.s. |
| Disease duration (months) |
50.56 (±30.49) |
47.83 (±34.50) |
56.00 (±22.10) |
n.s. |
| Sub-type (P/C) |
7/11 |
3/9 |
4/2 |
n.s. |
| Cognitive function |
| MMSE (/30) |
26.25 (±6.92) |
27.50 (±1.72) |
24.17 (±11.41) |
n.s. |
| FAB (/18) |
15.31 (±1.78) |
15.00 (±1.63) |
15.60 (±2.19) |
n.s. |
| Basic respiratory and motor
function |
| %VC |
70.39 (±23.84) |
80.25 (±20.28) |
50.67 (±18.11) |
<0.01 |
| %FVC |
66.72 (±24.59) |
78.50 (±20.23) |
43.17 (±12.50) |
<0.01 |
| %PImax |
55.66 (±37.80) |
69.84 (±36.24) |
27.30 (±22.81) |
<0.05 |
| %PEmax |
41.82 (±26.40) |
58.26 (±19.64) |
23.52 (±12.74) |
<0.01 |
| H&Y total |
4.05 (±0.99) |
3.67 (±0.98) |
4.83 (±0.41) |
<0.01 |
| SARA (/40) |
21.67 (±7.87) |
18.50 (±6.02) |
28.00 (±7.67) |
<0.05 |
| MDS-UPDRS Part 3 (/132) |
53.22 (±24.51) |
42.42 (16.95) |
74.83 (23.86) |
<0.01 |
| UMSARS Part 1 (/48) |
26.06 (±9.42) |
21.33 (±6.47) |
35.50 (±7.01) |
<0.01 |
| UMSARS Part 2 (/56) |
25.50 (±11.15) |
20.92 (±9.27) |
34.67 (±9.10) |
<0.01 |
| UMSARS Part 4 (/5) |
3.00 (±1.19) |
2.41 (±0.90) |
4.17 (±0.75) |
<0.01 |
Data are presented as number or mean (±SD).
BMI, body mass index; Sub-type P, Parkinsonian; Sub-type C, cerebellar; n.s.,
not significant.
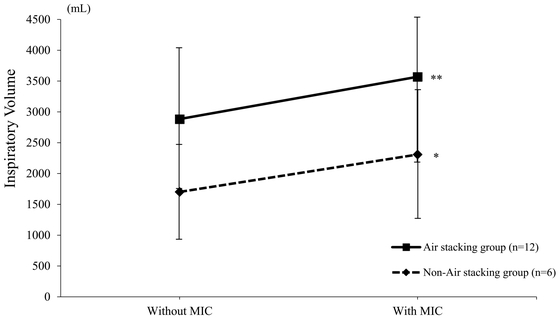
Figure 1 shows the inspiratory volume
changes with the use of MIC in both groups. In the air stacking group (squares), the
mean inspiratory volume without MIC was 2884.2 (± 1138.4) mL and that with MIC was
3570.8 (± 1174.6) mL. The application of MIC significantly increased inspiratory
volume (P < 0.01). In the non-air stacking group (diamond symbols), the mean
inspiratory volume without MIC was 1701.7 (± 769.3) mL and that with MIC was 2308.3
(± 1040.4) mL. Similarly, the respiratory volume was significantly increased with
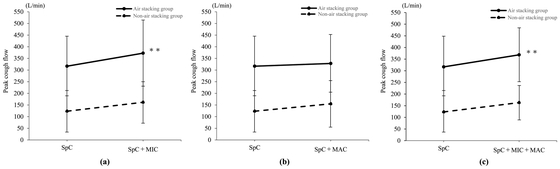
the use of MIC (P < 0.05). Figure 2
shows the changes in PCF with application of MIC and MAC. Figure 2a shows that PCF in the air stacking group (solid
line) was significantly increased with the use of MIC (from 316.7 ± 129.0 L/min to
372.5 ± 141.9 L/min, P < 0.01). In the non-air stacking group (dotted line), the
use of MIC increased PCF from 123.3 ± 89.1 L/min to 161.7 ± 88.9 L/min, but the
increase was not significant (P = 0.13). Figure
2b shows the changes of PFC with the use of MAC. Application of MAC in
the air stacking group (solid line) produced no significant difference (from
316.7 ± 129.0 L/min to 328.3 ± 123.6 L/min, P = 0.73). Similarly, the use of MAC in
the non-air stacking group (dotted line) showed no significant difference (from
123.3 ± 89.1 L/min to 155.0 ± 100.1 L/min, P = 0.25). Figure 2c shows the change of PCF when both MIC and MAC
were applied. This combined application of MIC and MAC significantly increased PCF
in the air stacking group (solid line) (from 316.7 ± 129.0 L/min to 368.3 ± 116.6
L/min, P < 0.01). However, this combined application did not produce a
significant increase of PCF in the non-air stacking group (dotted line) (from
123.3 ± 89.1 L/min to 163.3 ± 74.2 L/min, P = 0.11).
The results of ROC analysis are presented in Fig.
3.Figure 3a shows that the %FVC
cut-off value for discriminating SpC below 270 L/min was 74.5% (AUC = 0.852,
Sensitivity = 0.778, Specificity = 0.889, P < 0.05), and Fig. 3b shows that the %FVC cut-off value for
discriminating SpC below 160 L/min was 59.0% (AUC = 0.958, Sensitivity = 0.917,
Specificity = 1.0, P < 0.01). Similarly, the %FVC cut-off value for
discriminating SpC + MIC below 270 L/min was 70.5% (AUC = 0.935,
Sensitivity = 0.818, Specificity = 1.0, P < 0.01, Fig. 3c), and the %FVC cut-off value for discriminating
SpC + MIC below 160 L/min was 59.0% (AUC = 0.946, Sensitivity = 0.786,
Specificity = 1.0, P < 0.01, Fig.
3d).
DISCUSSION
To the best of our knowledge, this is the first report to clearly indicate that an
effective rehabilitative approach can improve cough function in MSA patients. First,
we observed that inspiratory volume was significantly increased by MIC. Second, we
found that PCF was successfully augmented by MIC in the air stacking group. Third,
it seemed difficult to maintain PCF at 160 L/min when %FVC fell below 59% even
though MIC was provided.
A previous study reported that MSA with predominant cerebellar ataxia was more common
than Parkinsonian type MSA in Japan.27) In addition, there was no significant difference
after a disease duration of approximately 5 years when patients with MSA were
divided into those with and without vocal cord dysfunction.28) Therefore, MSA
patients in this study are equivalent to those in natural history studies.
This study revealed that the inspiratory volume in patients with MSA could be
significantly increased by applying MIC (Fig.
1). In the cough function of healthy subjects, deep inspiration before
coughing reaches 85%–90% of the inspiratory volume, and a total cough volume of
about 2.3 L is reached to achieve sufficient PCF.29) In addition, a pre-cough lung volume
is essential to generate an effective cough in patients with neuromuscular disease
whose inspiratory and expiratory muscles are severely impaired.30) At the time of this
study, %VC, %FVC, %PImax, and %PEmax had already decreased in both groups (Table 1). For patients with MSA who have
difficulty achieving sufficient deep inspiration without assistance, application of
MIC may be recommended as an essential method to facilitate deep inspiration before
coughing.
PCF was found to be significantly improved by providing MIC (Fig. 2). However, in the non-air stacking group, various
cough assistance methods including MIC and MAC did not lead to a significant
increase in PCF. Improvement in PCF with the use of MIC is likely caused by
lengthening of the expiratory muscles by sufficient inspiration (length–tension
relationship), which increases intrathoracic pressure and leads to strong
PCF.25) In this
study, expansion of the expiratory muscle group and increase in intrathoracic
pressure may be caused by MIC. However, even in the air stacking group, MAC alone
did not improve PCF. A study investigating the limitations of cough augmentation
found MAC to be ineffective in patients with neuromuscular disease with a VC greater
than 1910 mL.31) For
patients in this study who could achieve a sufficient inspiratory volume, it was
considered that compression of the thorax alone would not lead to PCF improvement.
In the non-air stacking group, cough augmentation with MIC and/or MAC did not
improve PCF. By applying positive pressure, it was possible to achieve deep
inspiration greater than the VC. However, the inability of some patients to hold
their breath may have rendered any increase in intrathoracic pressure as
insufficient because of air leakage. Therefore, in MSA patients with insufficient
ability to hold their breath, it may be difficult to increase PCF with MIC and/or
MAC.
According to the ROC curve results, when %FVC is 74.5% or lower, spontaneous cough
strength is less than 270 L/min. However, by adding MIC to assisted coughing, the
cut-off value was maintained at 70.5%, indicating that effective expectoration could
be extended to that point. Previous studies reported that MIC is improved by
intervention even though VC decreases as the disease progresses.32) Increasing the
pre-cough inspiratory volume by MIC may extend the period of clinically effective
sputum expectoration. However, when %FVC is 70.5% or lower even with MIC, PCF
becomes less than 270 L/min. In general, when PCF falls below 270 L/min, other
coughing methods should be considered.15,24) Therefore, when %FVC falls below 70.5%, the methods
of cough augmentation performed in this study are not effective, and other cough
augmentation methods such as the mechanical insufflation-exsufflation need to be
considered. Furthermore, it was found that PCF could not be maintained at 160 L/min
when %FVC fell below 59% even when MIC was performed. It is known that PCF below 160
L/min signifies insufficient secretion clearance, and endotracheal intubation may be
necessary.24,25,26) Patients with MSA present with dysphagia.6) Swallowing difficulty
can lead to pneumonia and suffocation. Gastrostomy tube feeding is usually performed
for MSA patients showing repeated episodes of aspiration.6) From the results of this study, the
introduction of gastrostomy tube feeding for patients with MSA should be considered
when the cough function and respiratory function are insufficient.
This study has some limitations. First, the total of 18 out of 24 consecutive
patients who participated in this study was a small sample size. There are
approximately 12,000 MSA patients in Japan. Because MSA is a rare disease, the
sample size in previous studies on MSA in Japan was generally in the range of 20 to
50 people over a 5-year research period. Considering the low prevalence of MSA in
Japan, the result of this study is clinically meaningful, although the sample size
for our study was small. Future studies should be performed with more participants
to improve the accuracy of research results. Second, this study was conducted as a
single-facility intervention. A single source of patients could be insufficient to
make a trial of viable size and may be associated with bias of the subjects and the
risk of overestimation of intervention results when compared with a multicenter
trial. In addition, after extensive research, we found no previous studies on
rehabilitative interventions for respiratory muscle strength or cough strength.
Given the rare nature of this disease, multicenter collaborative research should be
conducted in the future. Third, this study used a cross-sectional study design. In
future studies, it is desirable to assess the long-term effects of cough
augmentation techniques as applied in this study for MSA patients. In particular,
the effects of these techniques when they are applied repeatedly should be
clarified.
CONCLUSION
By applying positive pressure with MIC, it was possible to increase inspiratory
volume just before coughing and to enhance PCF. Furthermore, when %FVC was less than
59%, the effect of cough augmentation became insufficient. Therefore, the results of
this study indicate the performance cut-off when consideration should be given to
the necessity of securing safe nutritional intake via gastrostomy.
ACKNOWLEDGMENTS
The authors are grateful to the patients and their families for contributing to our
study. We thank Professor Shoji Tsuji for introducing us to the Japan MSA registry
and providing advice on our study. We also thank specially appointed Associate
Professor Jun Mitsui and specially appointed Assistant Professor Takashi Matsukawa
for their cooperation in patient recruitment and for their advice on our
research.
CONFLICTS OF INTEREST
Mieko Ogino received honoraria for lecturing from Takeda Pharmaceutical. The
remaining authors declare no conflict of interest.
REFERENCES
- 1. Palma JA, Norcliffe-Kaufmann L, Kaufmann
H: Diagnosis of multiple system atrophy. Auton Neurosci 2018;211:15–25.
PMID:29111419, DOI:10.1016/j.autneu.2017.10.007
- 2. Reddy K, Dieriks BV: Multiple system
atrophy: α-synuclein strains at the neuron-oligodendrocyte crossroad. Mol
Neurodegener 2022;17:77. PMID:36435784,
DOI:10.1186/s13024-022-00579-z
- 3. Gilman S, Low PA, Quinn N, Albanese A,
Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan
I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK: Consensus statement
on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–98.
PMID:10223419, DOI:10.1016/S0022-510X(98)00304-9
- 4. Low PA, Reich SG, Jankovic J, Shults CW,
Stern MB, Novak P, Tanner CM, Gilman S, Marshall FJ, Wooten F, Racette B,
Chelimsky T, Singer W, Sletten DM, Sandroni P, Mandrekar J: Natural history of
multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol
2015;14:710–719. PMID:26025783,
DOI:10.1016/S1474-4422(15)00058-7
- 5. Wenning GK, Geser F, Krismer F, Seppi K,
Duerr S, Boesch S, Köllensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia
MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T,
Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A,
del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel
R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G,
Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W, European Multiple System
Atrophy Study Group: The natural history of multiple system atrophy: a
prospective European cohort study. Lancet Neurol 2013;12:264–274. PMID:23391524,
DOI:10.1016/S1474-4422(12)70327-7
- 6. Calandra-Buonaura G, Alfonsi E,
Vignatelli L, Benarroch EE, Giannini G, Iranzo A, Low PA, Martinelli P, Provini
F, Quinn N, Tolosa E, Wenning GK, Abbruzzese G, Bower P, Antonini A, Bhatia KP,
Bonavita J, Pellecchia MT, Pizzorni N, Tison F, Ghorayeb I, Meissner WG, Ozawa
T, Pacchetti C, Pozzi NG, Vicini C, Schindler A, Cortelli P, Kaufmann H:
Dysphagia in multiple system atrophy consensus statement on diagnosis, prognosis
and treatment. Parkinsonism Relat Disord 2021;86:124–132. PMID:33839029,
DOI:10.1016/j.parkreldis.2021.03.027
- 7.Niederman MS, Cilloniz C: Aspiration
pneumonia. Rev Eso Quimioter 2022;35:73–77. PMID:35488832,
DOI:10.37201/req/s01.17.2022
- 8. Kikuchi K, Satake M, Terui Y, Kimoto Y,
Iwasawa S, Furukawa Y: Cough peak flow with different mechanically assisted
coughing approaches under different conditions in patients with neuromuscular
disorders. Phys Ther Res 2019;22:58–65. PMID:32015942,
DOI:10.1298/ptr.E9978
- 9. Toussaint M, Chatwin M, Gonzales J,
Berlowitz DJ, ENMC Respiratory Therapy Consortium: 228th ENMC International
Workshop: airway clearance techniques in neuromuscular disorders Naarden, The
Netherlands, 3–5 March, 2017. Neuromuscul Disord 2018;28:289–298. PMID:29395673,
DOI:10.1016/j.nmd.2017.10.008
- 10. Shimohata T, Aizawa N, Nakayama H,
Taniguchi H, Ohshima Y, Okumura H, Takahashi T, Yokoseki A, Inoue M, Nishizawa
M: Mechanisms and prevention of sudden death in multiple system atrophy.
Parkinsonism Relat Disord 2016;30:1–6. PMID:27103478,
DOI:10.1016/j.parkreldis.2016.04.011
- 11. Wang Y, Shao W, Gao L, Lu J, Gu H, Sun L,
Tan Y, Zhang Y: Abnormal pulmonary function and respiratory muscle strength
findings in Chinese patients with Parkinson’s disease and multiple system
atrophy—comparison with normal elderly. PLoS One 2014;9:e116123. PMID:25546308,
DOI:10.1371/journal.pone.0116123
- 12. Cortelli P, Calandra-Buonaura G,
Benarroch EE, Giannini G, Iranzo A, Low PA, Martinelli P, Provini F, Quinn N,
Tolosa E, Wenning GK, Abbruzzese G, Bower P, Alfonsi E, Ghorayeb I, Ozawa T,
Pacchetti C, Pozzi NG, Vicini C, Antonini A, Bhatia KP, Bonavita J, Kaufmann H,
Pellecchia MT, Pizzorni N, Schindler A, Tison F, Vignatelli L, Meissner WG:
Stridor in multiple system atrophy: consensus statement on diagnosis, prognosis,
and treatment. Neurology 2019;93:630–639. PMID:31570638,
DOI:10.1212/WNL.0000000000008208
- 13. Gilman S, Wenning GK, Low PA, Brooks DJ,
Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H,
Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P,
Seppi K, Vidailhet M: Second consensus statement on the diagnosis of multiple
system atrophy. Neurology 2008;71:670–676. PMID:18725592,
DOI:10.1212/01.wnl.0000324625.00404.15
- 14. American Thoracic Society/European
Respiratory Society: ATS/ERS statement on respiratory muscle testing. Am J
Respir Crit Care Med 2002;166:518–624. PMID:12186831,
DOI:10.1164/rccm.166.4.518
- 15. Chatwin M, Toussaint M, Gonçalves MR,
Sheers N, Mellies U, Gonzales-Bermejo J, Sancho J, Fauroux B, Andersen T, Hov B,
Nygren-Bonnier M, Lacombe M, Pernet K, Kampelmacher M, Devaux C, Kinnett K,
Sheehan D, Rao F, Villanova M, Berlowitz D, Morrow BM: Airway clearance
techniques in neuromuscular disorders: a state of the art review. Respir Med
2018;136:98–110. PMID:29501255, DOI:10.1016/j.rmed.2018.01.012
- 16. The Japanese Association of Rehabilitation
Medicine: Japanese guidelines for pulmonary rehabilitation of neuromuscular
disease and spinal cord injury. Tokyo: Kanehara; 2014.
- 17. Yorimoto K, Ariake Y, Saotome T,
Mori-Yoshimura M, Tsukamoto T, Takahashi Y, Kobayashi Y: Lung insufflation
capacity with a new device in amyotrophic lateral sclerosis: measurement of the
lung volume recruitment in respiratory therapy. Prog Rehabil Med
2020;5:20200011. PMID:32789279, DOI:10.2490/prm.20200011
- 18. Kan AF, Butler JM, Hutchence M, Jones K,
Widger J, Doumit MA: Teaching manually assisted cough to caregivers of children
with neuromuscular disease. Respir Care 2018;63:1520–1527. PMID:30254045,
DOI:10.4187/respcare.06213
- 19. Krismer F, Seppi K, Jönsson L, Åström DO,
Berger AK, Simonsen J, Gordon MF, Wenning GK, Poewe W, European Multiple System
Atrophy Study Group Natural History Study Investigators, : Sensitivity to change
and patient-centricity of the unified multiple system atrophy rating scale
items: a data-driven analysis. Mov Disord 2022;37:1425–1431. PMID:35332582,
DOI:10.1002/mds.28993
- 20. Goetz CG, Poewe W, Rascol O, Sampaio C,
Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD,
Seidl L, Movement Disorder Society Task Force on Rating Scales for Parkinson’s
Disease: Movement Disorder Society Task Force report on the Hoehn and Yahr
staging scale: status and recommendations. Mov Disord 2004;19:1020–1028.
PMID:15372591, DOI:10.1002/mds.20213
- 21. Martínez-Martín P, Rodríguez-Blázquez C,
Mario Alvarez , Arakaki T, Arillo VC, Chaná P, Fernández W, Garretto N,
Martínez-Castrillo JC, Rodríguez-Violante M, Serrano-Dueñas M, Ballesteros D,
Rojo-Abuin JM, Chaudhuri KR, Merello M: Parkinson’s disease severity levels and
MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat Disord
2015;21:50–54. PMID:25466406,
DOI:10.1016/j.parkreldis.2014.10.026
- 22. Belas dos Santos M, Barros de Oliveira C,
dos Santos A, Garabello Pires C, Dylewski V, Arida RM: A comparative study of
conventional physiotherapy versus robot-assisted gait training associated to
physiotherapy in individuals with ataxia after stroke. Behav Neurol
2018;2018:1–6. PMID:29675114, DOI:10.1155/2018/2892065
- 23. Lee KK, Davenport PW, Smith JA, Irwin RS,
McGarvey L, Mazzone SB, Birring SS, Abu Dabrh AM, Altman KW, Barker AF, Birring
SS, Blackhall F, Bolser DC, Brightling C, Chang AB, Davenport P, El Solh AA,
Escalante P, Field SK, Fisher D, French CT, Grant C, Harding SM, Harnden A, Hill
AT, Irwin RS, Iyer V, Kahrilas PJ, Kavanagh J, Keogh KA, Lai K, Lane AP, Lim K,
Madison JM, Malesker MA, McGarvey L, Murad MH, Narasimhan M, Newcombe P,
Oppenheimer J, Rubin B, Russell RJ, Ryu JH, Singh S, Smith MP, Tarlo SM,
Vertigan AE, CHEST Expert Cough Panel: Global physiology and pathophysiology of
cough: part 1: cough phenomenology—CHEST guideline and expert panel report.
Chest 2021;159:282–293. PMID:32888932,
DOI:10.1016/j.chest.2020.08.2086
- 24. Morrow B, Argent A, Zampoli M, Human A,
Corten L, Toussaint M: Cough augmentation techniques for people with chronic
neuromuscular disorders. Cochrane Database Syst Rev 2021;4:CD013170.
PMID:33887060
- 25. Voulgaris A, Antoniadou M, Agrafiotis M,
Steiropoulos P: Respiratory involvement in patients with neuromuscular diseases:
a narrative review. Pulm Med 2019;2019:1–11. PMID:31949952,
DOI:10.1155/2019/2734054
- 26. Bach JR, Saporito LR: Criteria for
extubation and tracheostomy tube removal for patients with ventilatory failure.
A different approach to weaning. Chest 1996;110:1566–1571. PMID:8989078,
DOI:10.1378/chest.110.6.1566
- 27. Watanabe H, Saito Y, Terao S, Ando T,
Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G:
Progression and prognosis in multiple system atrophy: an analysis of 230
Japanese patients. Brain 2002;125:1070–1083. PMID:11960896,
DOI:10.1093/brain/awf117
- 28. Higo R, Tayama N, Watanabe T, Nitou T,
Takeuchi S: Vocal fold motion impairment in patients with multiple system
atrophy: evaluation of its relationship with swallowing function. J Neurol
Neurosurg Psychiatry 2003;74:982–984. PMID:12810801,
DOI:10.1136/jnnp.74.7.982
- 29. Kang SW, Kang YS, Sohn HS, Park JH, Moon
JH: Respiratory muscle strength and cough capacity in patients with Duchenne
muscular dystrophy. Yonsei Med J 2006;47:184–190. PMID:16642546,
DOI:10.3349/ymj.2006.47.2.184
- 30. Park JH, Kang SW, Lee SC, Choi WA, Kim
DH: How respiratory muscle strength correlates with cough capacity in patients
with respiratory muscle weakness. Yonsei Med J 2010;51:392–397. PMID:20376892,
DOI:10.3349/ymj.2010.51.3.392
- 31. Toussaint M, Boitano LJ, Gathot V, Steens
M, Soudon P: Limits of effective cough-augmentation techniques in patients with
neuromuscular disease. Respir Care 2009;54:359–366.
PMID:19245730
- 32. Kang SW, Bach JR: Maximum insufflation
capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med
Rehabil 2000;79:222–227. PMID:10821306,
DOI:10.1097/00002060-200005000-00002