2023 Volume 8 Article ID: 20230040
2023 Volume 8 Article ID: 20230040
Background: Magnetic stimulation devices can be large because of the need for cooling systems. We developed a compact and lightweight Spinning Permanent Magnet (SPM) device that generates magnetic fields with intensities below the motor threshold. In this report, we present the case of a post-stroke patient in which an immediate reduction in spasticity of the ankle plantar flexors was achieved after SPM treatment.
Case: A 37-year-old man was admitted to our hospital with a right putamen hemorrhage. The patient underwent conservative therapy and exhibited residual left hemiplegia and spasticity. Three months after stroke onset, he was able to walk with supervision while using a left ankle–foot orthosis and a T-cane. The Modified Ashworth Scale (MAS) score of the left ankle plantar flexors was 1+. The plantar flexors were stimulated by SPM treatment. The outcomes were the Hmax/Mmax of the tibial nerve (soleus muscle) and the MAS score. On the first day, SPM stimulation was applied for 30 min. On the second day, a sham stimulation of the same duration was performed. On the third day, the SPM stimulation was repeated. Hmax/Mmax decreased from 41.5% to 37.7% on the first day, and from 46.9% to 31.6% on the third day after SPM stimulation. The MAS score decreased from 1+ to 1 on both days. In contrast, after sham stimulation, Hmax/Mmax increased from 39.2% to 44.2%, whereas the MAS score remained unchanged at 1+.
Discussion: Stimulation below the motor threshold using SPM treatment can effectively reduce spasticity.
Spasticity is a sensorimotor control disorder that results from an upper motor neuron (UMN) lesion that presents as intermittent or sustained involuntary muscle activation.1) It is a common complication of stroke2) and it can have a disabling effect on stroke survivors because of pain and reduced mobility, which may limit the potential success of rehabilitation.3)
A systematic review of non-pharmacological interventions for spasticity in adults showed moderate evidence for electro-neuromuscular stimulation and acupuncture as adjunctive therapies to conventional routine care (pharmacological and rehabilitation) in post-stroke patients.4) In addition, transcutaneous electrical stimulation (TENS), which provides stimulation below the motor threshold, effectively reduces spasticity.5) The 2021 Japanese Guidelines for the Management of Stroke recommended TENS as a Grade A treatment for spasticity in patients with stroke, along with botulinum toxin A injection, phenol block, brace treatment, intrathecal baclofen, and oral muscle relaxants as Grade B treatments.6) However, TENS requires an electrode attachment, which can cause skin irritation.
Peripheral magnetic stimulation (PMS) can stimulate target nerves and muscles without using electrodes or clothing. PMS of the extremities is also effective in reducing spasticity even after a single session.7) Furthermore, PMS has the added advantage of causing less pain than electrical stimulation, because it does not directly stimulate pain receptors in the skin.8) PMS devices are typically bulky; however, devices delivering stimulation intensity below the motor threshold can be downsized. In this report, we present a case in which we observed immediate reduction in the spasticity of the ankle plantar flexors in a post-stroke patient after treatment with a Spinning Permanent Magnet (SPM) device.
SPM DeviceWe have developed a compact and lightweight magnetic stimulation device (SPM device), which generates magnetic fields by spinning a disc-shaped permanent magnet with a motor (Fig. 1).9) It can easily be attached to a limb using a Velcro strap.
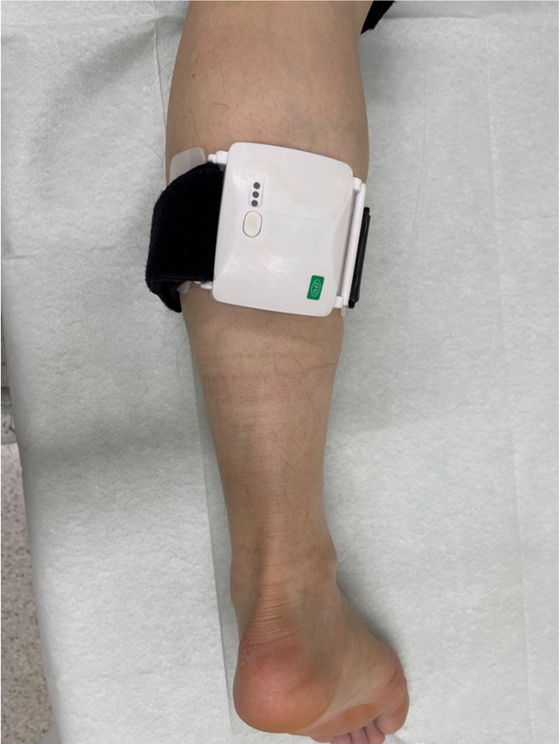
Photograph of the spinning permanent magnet (SPM) device attached to the patient’s lower leg. The device is compact and lightweight, measuring 7.5 × 6.3 × 2.5 cm and weighing 165 g. It can easily be attached to limbs using the Velcro strap.
This study was approved by the Fujita Health University Certified Review Board and was registered with the Japan Registry of Clinical Trials (Registration No. jRCTs042200013). Written informed consent was obtained from the patient for use of the SPM device and for publication of this case report. This study conformed to the CARE guidelines.10)
A 37-year-old man who had suffered a right frontal subcortical hemorrhage was admitted to our hospital on the day of onset in 2021. Figure 2 shows a computed tomography scan of the head. The patient had kidney cancer and was taking antineoplastic medications. Conservative treatment was undertaken for subcortical hemorrhage, and rehabilitation was initiated on the day of onset with 100–120 min of physical therapy and 40–60 min of occupational therapy, five times a week. The patient had left hemiplegia, and a left ankle–foot orthosis was fabricated approximately 7 weeks after onset. The orthosis was a shoehorn brace that controlled ankle dorsiflexion and plantar flexion by utilizing the flexibility of plastic. The initial dorsiflexion angle of the brace was set at 5 degrees. A WalkAide® device (Innovative Neurotronics, Austin, TX, USA) was also prescribed approximately 9 weeks after onset. The patient engaged in walking exercises with both the orthosis and the WalkAide to enhance ankle stability and activate ankle dorsiflexion. Three months after onset, he was able to walk with the support of the orthosis and a T cane. He discontinued the use of the WalkAide at this time because he did not have any significant ankle functional improvement after 3 weeks and we believed that an increase in walking could potentially yield further functional improvements in the future. The patient gave the following Stroke Impairment Assessment Set motor item (SIAS-M) scores: hip flexion, 2; knee extension, 2, and foot-tap test, 1. Spasticity of the left limbs developed gradually, and the Modified Ashworth Scale (MAS) score of the left ankle plantar flexors was 1+. The dorsiflexion angle was 5 degrees with the knee extended, and the ranges of motion of other joints in the lower extremity were normal. Deep tendon reflexes of the left side increased, and left ankle clonus was sustained for more than 30 s. The Manual Muscle Test score for the right lower extremity was 5.

Computed tomography scan of the patient’s head shows a right frontal subcortical hemorrhage.
The Functional Independence Measure scores were 73 for motor function and 35 for cognition. Thirteen weeks after onset, use of the SPM was initiated to reduce the spasticity of the patient’s left ankle plantar flexors.
The left ankle plantar flexors were stimulated in the prone position with the SPM device placed on the posterior portion of the lower leg at the point of maximum circumference (see Fig. 1). On the first day, SPM stimulation was applied for 30 min. On the second day, the device was attached to the same location but was not turned on (sham stimulation). On the third day, the SPM stimulation was repeated. The primary outcomes were the maximal amplitude of the H-reflex as a percentage of the maximal M response (Hmax/Mmax) of the tibial nerve (soleus) and the MAS score. The secondary outcome was the 10-m walking time when walking with a T-cane and ankle–foot orthosis. Electromyography was performed using a Neuropack X1 MEB-2300 (Nihon Kohden, Tokyo, Japan). The recording electrode was placed on the left soleus, the reference electrode was located 5 cm distal to it, and stimulation was performed at the popliteal fossa. We also recorded and analyzed gait while the patient walked with the T-cane but without the orthosis. Evaluations were conducted three times daily: before stimulation, immediately after stimulation, and 24 h after stimulation.
On the first day after stimulation, Hmax/Mmax decreased from 41.5% to 37.7%, and the MAS score decreased from 1+ to 1 (Fig. 3 and Table 1). On the second day, both Hmax/Mmax and the MAS score were increased before stimulation. After sham stimulation, Hmax/Mmax increased from 39.2% to 44.2% and the MAS score remained unchanged at 1+. On the third day after stimulation, Hmax/Mmax decreased from 46.6% to 30.3% and the MAS score decreased from 1+ to 1. Hmax/Mmax and MAS score increased to 44.2% and 1+, respectively, on the fourth day. The 10-m walk time with the T-cane and orthosis showed a trend of improvement on all days: on the first day, it was 11.6 s before stimulation, 11.34 s immediately after stimulation, and 11.22 s at 24 h after stimulation. On the second day, it was 11.07 s immediately after stimulation and 10.95 s at 24 h after stimulation. On the third day, it was 10.03 s immediately after stimulation and 9.74 s at 24 h after stimulation.
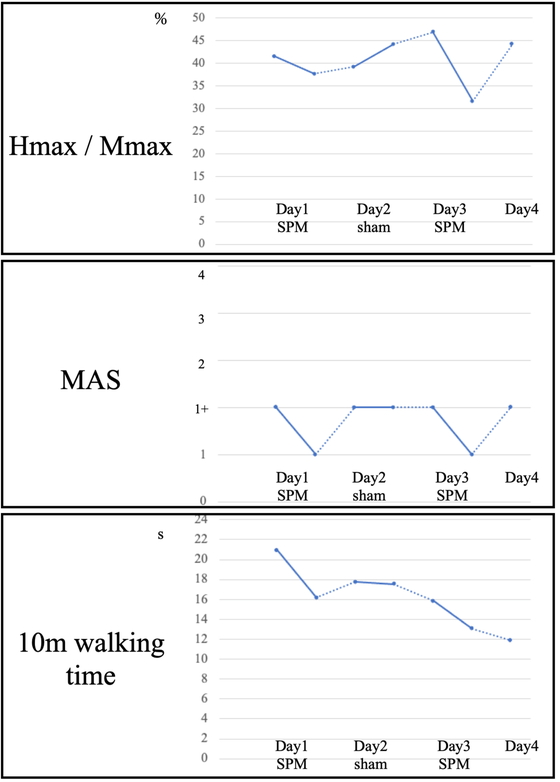
Timelines of Hmax/Mmax (top), MAS score (middle), and 10-m walking time (bottom) when walking with a left ankle–foot orthosis and a T-cane. Solid lines indicate SPM or sham stimulation.
| Day 1 | Day 2 | Day 3 | Day 4 | ||||
| Time course (h) | 0 | 0.5 | 24 | 24.5 | 48 | 48.5 | 72 |
| Pre-stim | Post-stim | Pre-stim | Post-stim | Pre-stim | Post-stim | Pre-stim | |
| SPM | Sham | SPM | |||||
| Hmax/Mmax (%) | 41.5 | 37.7 | 39.2 | 44.2 | 46.6 | 30.3 | 44.2 |
| H-amplitude max (mV) | 9.5 | 9.2 | 9.2 | 10.9 | 9.7 | 6.4 | 8.6 |
| M-amplitude max (mV) | 22.9 | 24.5 | 23.5 | 24.6 | 20.8 | 21.0 | 19.4 |
Pre-stim, before stimulation; Post-stim, immediately after stimulation.
A video recording of the gait with a T-cane and without the orthosis before stimulation on the first day (Movie 1, Suppl. Video 1) is shown as part of Fig. 4. The video shows that initial foot contact was with the forefoot. However, Movie 2 (Fig. 4, Suppl. Video 2), which was recorded after stimulation, shows initial foot contact on the heel. Moreover, 24 h after stimulation on the first day, the foot-tap test score on the SIAS-M increased from 1 to 2, and the score continued to be 2 until the end of the study.

QR codes to video recordings of patient walking with a T-cane without the orthosis before SPM stimulation on Day 1 (Movie 1, left, Suppl. Video 1) and the patient walking with a T-cane without the orthosis after SPM stimulation on Day 1 (Movie 2, right, Suppl. Video 2).
After the conclusion of this study, the patient was discharged to another hospital for further evaluation of his kidney cancer and was readmitted to our hospital for additional cancer treatment. The patient experienced recurrent cranial hemorrhage because of brain metastasis, which worsened the left hemiplegia. Despite this, rehabilitation with magnetic stimulation was continued, and botulinum toxin A was injected to treat the remaining spasticity in the left tibial posterior, gastrocnemius, and soleus muscles. The patient was discharged and was able to walk independently with the orthosis and T-cane at 8 months after onset. No adverse effects were detected with the long-term use of magnetic stimulation using the SPM device.
We present a case in which immediate reduction in spasticity in the ankle plantar flexors of a stroke patient was observed after treatment with an SPM device. This compact device, which provides magnetic stimulation below the motor threshold, has not been described in previous studies. The patient’s spasticity improved immediately following SPM stimulation, as evidenced by favorable changes in Hmax/Mmax and the MAS score and observed improvements in gait.
In clinical practice, Hmax/Mmax is often used as a reliable indicator of spasticity.11) Hmax represents the number of excited alpha motor neurons in the anterior horn of the spinal cord when the input from group Ia fibers is maximized through electrical stimulation. In contrast, the Mmax indicates the amplitude of the muscle action potential when all the alpha motor neurons dominating the muscle are synchronously excited. Therefore, the Hmax/Mmax reflects the fraction of excited alpha motor neurons among all alpha motor neurons that dominate the target muscle during electrical stimulation. According to a previous study among 24 stroke patients and 12 age-matched healthy individuals, the mean Hmax/Mmax of the tibial nerve on the affected side, with the patient in the prone position, was higher in stroke patients (37.95% ± 15.16%) than in healthy individuals (26.88% ± 11.88%).12) The Hmax/Mmax of our patient’s affected spastic leg was similar to that of stroke patients in a previous study, although it improved to some degree after SPM stimulation. We speculate that the Hmax/Mmax observed before stimulation on Day 3 was higher than that observed before stimulation on Day 1 because of a decrease in the M wave. Although we made efforts to place the electrode in the same position and each examination was conducted with supramaximal stimulation, some factors such as muscle fatigue or body temperature also affect the height of the M wave.13) Such effects need to be carefully considered.
The phenomenon of spasticity is attributed to the hyperexcitability of the stretch reflex mediated by Ia afferents, which is caused by loss of inhibition in the dorsal reticulospinal tract.14) We believe that the underlying mechanism responsible for the effect of SPM is presynaptic inhibition of hyperactive stretch reflexes and reduced co-contraction of spastic antagonist muscles, which is similar to the effect of TENS.15)
The use of SPM treatment has several advantages. The SPM device does not produce a tingling sensation because it does not stimulate the skin pain receptors, as is the case with PMS. Another advantage of SPM treatment is its ease of use. The device is lightweight with a rechargeable battery and can be easily attached to limbs using a Velcro strap. This allows patients with the SPM device attached to their limbs to perform exercises in relative comfort.
This study was limited in that it involved a single case and showed only the immediate effects of SPM treatment. Therefore, the long-term effects of the treatment should be evaluated in a large sample size.
We report a post-stroke case in which an immediate reduction in ankle plantar flexor spasticity was achieved after treatment with an SPM device. Our findings suggest that magnetic stimulation using the SPM device, which provides stimulation below the motor threshold, may be useful for immediate reduction of spasticity.
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant No. JP21K17520). This case report was presented at the 6th autumn meeting of the Japanese Association of Rehabilitation Medicine in Okayama, Japan, on November 4, 2022. We thank Editage (www.editage.com) for English language editing of a draft version of this report.
Hitoshi Kagaya is listed as an inventor on a patent pending for the SPM device. The remaining authors declare no conflict of interest.