2023 Volume 8 Article ID: 20230041
2023 Volume 8 Article ID: 20230041
Background: Aphasia is a common disorder among stroke patients. Assessment of aphasia is essential for scheduling appropriate rehabilitative treatment. Although this is conventionally accomplished using neuropsychological test batteries, these tests are not always accessible because of attention and/or consciousness disturbances during acute care. To overcome this issue, we have introduced a newly developed automated tractography known as XTRACT.
Cases: Diffusion-tensor images were acquired from three patients on days 10–14. Brain images were processed by XTRACT, which automatically extracts neural tracts using standardized protocols. Fractional anisotropy (FA) values were then bilaterally evaluated in the following neural tracts associated with aphasia: arcuate fasciculus, inferior fronto-occipital fasciculus, middle longitudinal fasciculus, inferior longitudinal fasciculus, and uncinate fasciculus. Case 1 had word-finding difficulty on admission. FA values in the lesioned left hemisphere were not decreased in all tracts and this patient fully recovered during acute care. Case 2 had reduced spontaneous speech and a low FA value in the left arcuate fasciculus. Rehabilitative treatment was scheduled to improve the verbal output of sentences and word recall. Case 3 could not complete the conventional aphasia test battery because of attention disturbance. He had low FA values in all tracts in the left hemisphere. Rehabilitative treatment was designed to focus on both speaking and auditory comprehension.
Discussion: Automated tractography enables quantitative assessment of the neural damage associated with aphasia, even in patients with attention and/or consciousness disturbances. This modality can aid in the assessment of aphasia and allows the planning of appropriate rehabilitative treatment.
Aphasia is a major disorder in patients with acute stroke.1) For stroke patients with aphasia, a detailed assessment of symptoms is essential for appropriate rehabilitative treatment.2) In the clinical setting, this assessment is conventionally undertaken using neuropsychological test batteries for language function, including the Western Aphasia Battery3) and the Standard Language Test for Aphasia (SLTA).4,5) However, these test batteries require patients’ arousal and concentration, which may be difficult in acute care stroke patients, especially those with cognitive decline.6) In such cases, objective methods for assessing aphasia are desirable.
Magnetic resonance diffusion-tensor imaging (DTI) is a unique technique that can be used to assess neural fibers.7,8) Several studies have reported that DTI tractography could be useful in the assessment of aphasia.9,10) However, the vast majority of existing DTI studies focused on aphasia used manually defined tractography, which has lower reproducibility and requires time-consuming labor.11) Partially because of this burden, DTI tractography has not been widely used in real-world clinical settings. Recently, a novel automated tractography known as XTRACT was developed, which uses standardized protocols for extracting neural tracts in an automated manner.12,13) In this report, we describe the clinical utility of XTRACT for assessment of aphasia in acute care stroke rehabilitation.
This case series report includes the cases of three stroke patients with aphasia (Table 1) who were admitted to Nishinomiya Kyoritsu Neurosurgical Hospital, Japan. The study protocol was approved by the Ethics Committee of Nishinomiya Kyoritsu Neurosurgical Hospital (approval date: June 20, 2022) and the Hyogo Medical University Ethics Committee (approval number 4453). The patients were admitted to our hospital after stroke onset and underwent MRI examination. As standard protocol for patients with suspected stroke, diffusion-weighted imaging (DWI) was conducted.14) All three patients were right-handed and none had neurological disorders before onset. Written informed consent was obtained from the patients or their family for publication of this report.
| Characteristic | Case 1 (81 years, female) | Case 2 (53 years, male) | Case 3 (67 years, male) |
| Type and severity of aphasia | Mild motor aphasia | Moderate motor aphasia | Severe sensory aphasia |
| Other symptoms | Mild right hemiparesis | None | Attention disorder |
| Site of lesion | Corona radiata | Parietal and temporal lobes | Temporal lobe |
| Stroke treatment | Antiplatelet therapy | Antiplatelet therapy | Anticoagulant therapy |
| Focus of aphasiatreatment | Verbal output of sentences, word recall | Verbal output of sentences, word recall | Speaking, word comprehension |
| DTI acquisition time | Day 11 | Day 14 | Day 10 |
The stroke treatment protocol was applied in line with the recommendations of the Japanese Guidelines for the Management of Stroke 2021.15) In addition to conservative treatments such as anticoagulant or antiplatelet medication, the patients received rehabilitation comprising physical, occupational, and speech therapy for a combined duration of up to 180 min per day. For the clinical examination of aphasia, the SLTA was used; this is the most widely used neuropsychological test battery for aphasia in Japan.4,5) This test battery has 26 subtests for the evaluation of hearing, speaking, reading, writing, and calculation.
DTI Acquisition and Image ProcessingThe details of DTI acquisition and image processing methods are described in our previous report.13) In brief, diffusion-tensor MRI images were acquired in the second week after admission to our hospital using a 3.0-T scanner (MAGNETOM Trio; Siemens AG, Erlangen, Germany) with a 32-channel head coil. Because DTI detects signal changes caused by Wallerian degeneration following stroke, we obtained DTI on the second week when reliable signal changes were expected to occur.16) A single-shot echo-planar imaging sequence was used to acquire the DTI data; it comprised 30 images with non-collinear diffusion gradients (b=1500 s/mm2) and five non-DWI scans (b=0 s/mm2). For image acquisition, 80 contiguous axial slices were acquired from each patient using the following parameters: acquisition matrix, 128 × 128; field of view, 256 × 256 mm; slice thickness, 2 mm; repetition time, 10,900 ms; echo time, 96 ms; and flip angle, 90°. A three-dimensional fast gradient imaging sequence was also used to acquire T1-weighted images. In total, 176 contiguous sagittal slices were acquired from each patient: acquisition matrix, 256 × 256, field of view, 256 × 256 mm; slice thickness, 1 mm; repetition time, 2.52 ms, echo time, 1900 ms; and flip angle, 10°. The total duration time for image acquisition was 20 min.
For image processing, we used MRtrix17) and the FMRIB Software Library (FSL).18) To obtain probabilistic tractography based on the diffusion-tensor model, we applied the XTRACT tool12) in FSL. Parameter estimates, such as fractional anisotropy (FA) values and tract volumes, were extracted by the “xtract_stats” command in FSL. Based on our previous study,13) the threshold was set at 0.01. For the current study, we set the analytical target to the neural tracts associated with aphasia symptoms; these tracts included the arcuate fasciculus (AF), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), middle longitudinal fasciculus (MdLF), and uncinate fasciculus (UF).19,20,21) These tracts were assessed bilaterally.
To assess the damage to the neural tracts, the distribution of FA values in the non-lesioned hemispheres for the five tracts was investigated for normative reference. Data were obtained from the patients admitted to Nishinomiya Kyoritsu Neurosurgical Hospital for stroke between April 2022 and March 2023. Informed consent was obtained by the opt-out method. The patients were limited to first-ever stroke with supratentorial and unilateral lesion in the cerebral hemisphere. All included patients were functionally independent in activities of daily living before stroke onset. FA values were obtained from the non-lesioned hemisphere. For this analysis, we used 43 patients with right hemisphere lesions and 35 patients with left hemisphere lesions. The mean and standard deviation data for the five tracts are shown in Table 2. For assessment of the neural tracts of the patients in this case report, tracts with FA values that were smaller than the mean value in Table 2 by more than one standard deviation (in the bottom 15% of a normal distribution) were regarded as potentially damaged. Considering that the nature of the current study is the screening of damage within the neural tracts, such liberal thresholding is considered appropriate.
| Tract | Right hemisphere | Left hemisphere |
| AF | 0.465±0.039 | 0.470±0.039 |
| IFOF | 0.492±0.029 | 0.482±0.039 |
| MdLF | 0.451±0.030 | 0.429±0.032 |
| ILF | 0.438±0.036 | 0.423±0.035 |
| UF | 0.431±0.028 | 0.410±0.045 |
Data presented as mean ± standard deviation. Data for right hemisphere derived from 35 patients with left hemisphere lesions (age range 46–88 years); data for left hemisphere obtained from 43 patients with right hemisphere lesions (age range 42–89 years).
The patient was an 81-year-old woman (Table 1) who was transferred to our acute care service because of sudden onset of right hemiparesis and dysarthria. DWI showed high-intensity areas in the left corona radiata (Fig. 1). She was treated conservatively with antiplatelet medication. She had mild hemiparesis in the extremities (Brunnstrom recovery stage,22,23) V-V-V) and showed signs of mild motor aphasia; she was able to auditorily comprehend sequential commands but had apraxia of speech and word-finding difficulty when communicating with medical staff. Further examination of the patient’s aphasia using the SLTA showed low scores for “animal category fluency,” in which she was able to verbally list only three animal names in 1 min (Fig. 2). We focused her aphasia training on the verbal output of sentences and word recall. Tractography images were visually inspected for the location of the targeted tracts (Fig. 3). The FA values obtained from automated tractography were not decreased in the left damaged hemisphere (Table 3). The neural tracts associated with aphasia were mostly intact. We therefore assumed that her aphasia symptoms would recover quickly. Indeed, after completion of treatment in the acute phase, she recovered her ability to speak fluently and was discharged home on day 15.
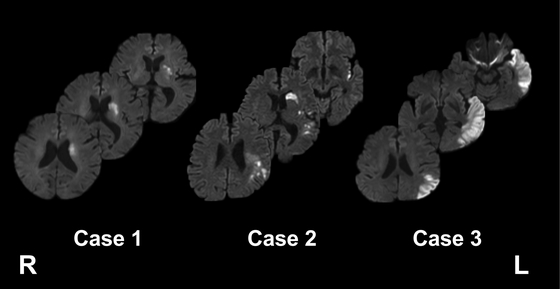
Diffusion-weighted magnetic resonance images for the three patients in this report. L, left; R, right.

Standard Language Test for Aphasia (SLTA) scores for the three patients in this report. The vertical axis indicates the correct rate (percentage) for each subtest in the SLTA. The English translations5) for the subtests are as follows: 1, Auditory word recognition; 2, Auditory sentence comprehension; 3, Verbal sequential commands; 4, Speech sound–kana letter choice matching; 5, Picture naming; 6, Word repetition; 7, Action naming; 8, Picture story description; 9, Sentence repetition; 10, Animal category fluency; 11, Oral reading of kanji word; 12, Oral reading of kana letter; 13, Oral reading of kana word; 14, Oral reading of sentence; 15, Written kanji word–picture choice matching; 16, Written kana word–picture choice matching; 17, Written sentence–picture choice matching; 18, Written sequential commands; 19, Writing kanji names of pictures; 20, Writing kana names of pictures; 21, Writing of picture story; 22, Writing kana letter to dictation; 23, Writing kanji word to dictation; 24, Writing kana word to dictation; 25, Writing dictated sentence; 26, Calculation; I, Hearing; II, Speaking; III, Reading; IV, Writing; V, Calculation.

Three-dimensional images obtained by automated tractography. Yellow areas, AF; blue areas, IFOF; red areas, MdLF; green areas, ILF; purple areas, UF; L, left; R; right.
| Parameter | Tract | Case 1 | Case 2 | Case 3 | |||
| Right | Left | Right | Left | Right | Left | ||
| FA value | AF | 0.450 | 0.457 | 0.449 | 0.400 a | 0.491 | 0.410 a |
| IFOF | 0.486 | 0.482 | 0.482 | 0.466 | 0.484 | 0.427 a | |
| MdLF | 0.431 | 0.446 | 0.412 | 0.423 | 0.428 | 0.294 a | |
| ILF | 0.397 | 0.420 | 0.341 | 0.359 a | 0.395 | 0.203 a | |
| UF | 0.453 | 0.445 | 0.406 | 0.392 | 0.419 | 0.311 a | |
| Tract volume (ml) | AF | 2.640 | 2.240 | 1.952 | 1.744 | 2.328 | 2.960 |
| IFOF | 3.376 | 3.424 | 3.368 | 3.040 | 4.048 | 4.240 | |
| MdLF | 2.536 | 2.928 | 1.816 | 1.968 | 2.592 | 2.808 | |
| ILF | 1.632 | 1.832 | 1.112 | 1.640 | 1.848 | 2.168 | |
| UF | 1.880 | 2.112 | 1.328 | 1.432 | 1.920 | 1.560 | |
a FA value is smaller than the mean value for the left hemisphere (given in Table 2) by more than one standard deviation.
The patient was a 53-year-old man (Table 1) who was transferred to our acute care service after sudden onset of difficulty speaking during morning assembly at work. DWI revealed diffuse high-intensity areas in the left middle cerebral artery region, mainly in the parietal and temporal lobes (Fig. 1). He was treated conservatively with antiplatelet medication. The patient had no hemiparesis and had preserved auditory sentence comprehension, but he struggled to understand sequential commands. He seldom spoke spontaneously and was slow to respond when communicating with medical staff. The results from the SLTA showed low scores in “verbal sequential commands,” “picture naming,” “action naming,” “picture story description,” “sentence repetition,” and “animal category fluency” (Fig. 2). Tractography images were visually inspected (Fig. 3), and FA evaluation indicated a low value in the left AF and ILF (Table 3). The FA decrease in the ILF was also observed in the right hemisphere. Accordingly, we did not consider the FA decrease in the left ILF as pathological. Therefore, we focused his training on the verbal output of sentences and word recall. After completion of the acute-phase treatment, the patient showed improvements in spontaneous speech but still struggled with word recall and had paraphasia. He was transferred to a rehabilitation hospital on day 29 to continue rehabilitative treatment for aphasia.
Case 3The patient was a 67-year-old man (Table 1) who visited our hospital after his wife noticed a sudden onset of inconsistency between his words and actions. DWI revealed high-intensity areas in the left temporal lobe (Fig. 1). He was treated conservatively with anticoagulant medication. No hemiparesis was noted, and his gait and hand dexterity were intact. He showed signs of sensory aphasia; he could not auditorily comprehend simple verbal commands, and, although his speech was fluent, he had phonological and verbal paraphasia. Therefore, it was difficult for the medical staff to understand his verbal speech. He could not concentrate and did not complete most of the subtests in the SLTA evaluation (Fig. 2). Tractography images were visually inspected for the targeted tracts (Fig. 3). FA evaluation gave low estimates in all five tracts associated with aphasia (Table 3). The results showed that his speech and auditory comprehension were severely damaged. Considering these results, we focused his rehabilitative treatment on both speaking and comprehension. In addition, we assumed that he would need continuous high-intensity rehabilitation for aphasia. Therefore, the patient was transferred to our affiliated rehabilitation hospital on day 17 to continue rehabilitative treatment.
This case series report describes the clinical utility of automated tractography in acute-phase stroke patients with aphasia. Three stroke patients with left hemisphere lesions with aphasia were examined, and a set of neural tracts associated with language functions were examined. The results showed that FA values were decreased in line with clinical symptoms, such as aphasia severity and type. These findings suggest that automated tractography can be applied for the assessment of aphasia in clinical settings during acute care. This modality may be especially useful in patients with attention and/or consciousness disturbances that prevent the use of conventional neuropsychological test batteries.
Automated tractography can support the conventional methodology for assessing aphasia in acute stroke patients. The assessment of aphasia by neuropsychological test batteries depends on the level of patient alertness. Indeed, a previous study reported that aphasia could not be assessed in 28% of patients using neuropsychological test batteries within 7 days after stroke.6) In contrast, automated tractography results can be obtained whenever patients can undergo magnetic resonance imaging. In Case 3, the patient was unable to concentrate when communicating with medical staff. Consequently, we could not assess most of the SLTA subtests. In contrast, the results from automated tractography acquired in the second week showed decreased FA values in all five tracts in the lesioned left hemisphere. Overall, the patient was considered to have severe aphasia, which required further intensive inpatient rehabilitative treatment. Therefore, in cases where conventional neuropsychological test batteries are difficult, automated tractography can be an alternative for the assessment of aphasia.
Based on a comparison of aphasic patients and age-matched controls, our previous study showed that FA values were lower in the left AF in patients with aphasia following stroke.24) In contrast, the current study reports a detailed description of aphasia in relation to wider regions of neural structures associated with aphasia. From this perspective, the findings from Case 3 may suggest expansion of the use of FA to wider regions. In comparison with the FA norms for the five tracts associated with aphasia as in Table 2, the present findings imply the plausible use of this approach for quantitative assessment for aphasia. Such distribution patterns in the targeted neural tracts can be a useful index for the assessment of aphasia. Nonetheless, further studies are needed to expand on this topic.
Automated tractography may also be useful for defining the aphasia subtype in patients after stroke. It was previously proposed that language processing be modeled in two streams; the dorsal stream mainly comprises the AF and is responsible for mapping speech sounds to articulation, whereas the ventral stream mainly comprises the IFOF, MdLF, ILF, and UF and is responsible for mapping speech sounds to meaning.19,25,26) In a previous DTI study, language production was associated with the FA value of the left AF, and language comprehension was related with the FA value of the left IFOF and ILF.19) In Case 2, decreased FA values were observed in the AF, which is in the dorsal stream. This patient struggled with spontaneous speech but had largely preserved auditory comprehension. Meanwhile, Case 3 showed decreased FA values in the left AF, IFOF, MdLF, ILF, and UF, which are tracts in both the dorsal and ventral streams. This patient had both phonological and verbal paraphasia and also had dysfunctional auditory comprehension. Therefore, our results from automated tractography were compatible with those in the literature.19,25,26)
In addition to FA values, tract volumes were also obtained from our analyses. Unlike FA values, our analysis of the three cases in this case series did not reveal any apparent declines in tract volumes in the left hemisphere when compared with the right hemisphere. Currently, we are conducting preliminary analyses on correlations among FA values, tract volumes, and stroke outcomes for 78 stroke samples (as presented in Table 2). The results obtained so far indicate that FA values in specific tracts (e.g., the corticospinal tract) showed correlations (correlation coefficients >0.5) with outcomes such as hemiparesis and functional independence. However, no statistically significant findings were observed between tract volumes and outcomes. Further investigations are needed to address this matter thoroughly.
At our local community hospital, we use standardized automated tractography in daily clinical practice. Using UNIX shell script programming, the analyses are automatically performed with minimal manual labor. Recently, graphics processing units (GPUs) have become available for accelerating DTI analyses.27,28) With the GPU setting on our regular desktop computer (POWERSTEP Tower for Lin4Neuro; AMULET, Japan),29) only 40 min is required to complete the entire analytical process for each patient. Therefore, we can share the results of automated tractography with our medical staff soon after DTI acquisition. This technological advance allows wide application of automated tractography in real-world clinical settings.
There are several limitations to this study. First, FA values and tract volumes depend on the threshold setting (0.01). However, no consensus has been reached regarding this setting. Second, the relationship of the FA value obtained from automated tractography with the results of each subset of the SLTA remains to be clarified. Elucidation of this relationship will require the analysis of automated tractography in a greater number of stroke patients with aphasia. Third, in this study, we designated damaged neural tracts as those with FA values that were smaller than the mean value for the non-lesioned hemisphere by more than one standard deviation. Given that the focus of the present study was on detecting injuries in neural tracts, such liberal thresholding can be considered appropriate. Fourth, the long-term outcome of aphasia in relation to the FA values of neural tracts associated with aphasia remains unclear. Previous DTI studies have investigated the association between the superior longitudinal fasciculus and cognitive outcomes such as functional independence measures.30,31) Other tractography studies sought to predict language outcomes.9,32,33) Such knowledge is useful for predicting aphasia prognosis from acute care. Fifth, the usage of DTI to evaluate white matter tracts has methodological limitations. Crossing fiber regions can potentially result in misleading conclusions because of the limitation of estimating only a single fiber direction per voxel.34) Despite these limitations, the use of automated tractography in daily clinical practice is a novel and useful method for assessing aphasia in acute care stroke patients.
This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number JP22K11356) and by a Grant-in-Aid for Scientific Research on Innovative Areas—Platforms for Advanced Technologies and Research Resources (Advanced Bioimaging Support; JSPS KAKENHI Grant Number JP22H04926).
The authors declare no conflict of interest.