2025 Volume 11 Issue 1 Article ID: cr.24-0082
2025 Volume 11 Issue 1 Article ID: cr.24-0082
INTRODUCTION: The phosphatase and tensin homolog hamartoma tumor syndrome (PHTS) refers to a spectrum of disorders caused by variants of the phosphatase and tensin homolog (PTEN) gene, including Cowden syndrome (CS), Bannayan–Riley–Ruvalcaba syndrome, adult Lhermitte–Duclos disease, and autism spectrum disorders associated with macrocephaly. PHTS is characterized by hamartomas in multiple organs and is associated with an increased risk of developing malignant tumors including, breast, thyroid, endometrial, colorectal, and kidney tumors. Breast cancer is the most common malignancy associated with PHTS.
CASE PRESENTATION: We describe the case of a 44-year-old female patient with invasive ductal carcinoma of the right breast. Cobblestone papillomatosis was present in the gingiva. She had a medical history of bilateral adenomatous goiters for 10 years. Her mother had been diagnosed with breast cancer, thyroid and tongue tumors, gastric polyps, hepatic hemangioma, and collagen disease. Additionally, the patient’s maternal grandmother had a history of colon cancer. Based on the patient’s family history and physical findings, CS was suspected, and direct DNA sequencing analysis revealed a haplotype c.634del mutation in exon 7 of the PTEN gene. Although there is no clear evidence supporting risk-reducing surgery for PHTS, a right nipple-sparing mastectomy, sentinel lymph node biopsy, and tissue expander reconstruction were performed.
CONCLUSIONS: We report a case of breast cancer with a newly diagnosed c.634del mutation in the PTEN gene. We also reviewed the current literature on PTEN genetic variants and breast cancer subtypes.
National Comprehensive Cancer Network
PHTSPTEN hamartoma tumor syndrome
PTENphosphatase and tensin homolog
CSCowden syndrome
LDDLhermitte–Duclos disease
The phosphatase and tensin homolog hamartoma tumor syndrome (PHTS) refers to a spectrum of disorders caused by variants of the phosphatase and tensin homolog (PTEN) gene, including Cowden syndrome (CS), Bannayan–Riley–Ruvalcaba syndrome, adult Lhermitte–Duclos disease (LDD), and autism spectrum disorders associated with macrocephaly. CS is characterized by multiple hamartomas and carries a high risk of both benign and malignant tumors of the breast, thyroid, endometrium, colorectum, and kidneys.1) The most common malignancy associated with PHTS is breast cancer, with a reported lifetime risk of 85%.2) Here, we report a case of breast cancer with a novel mutation in the PTEN gene.
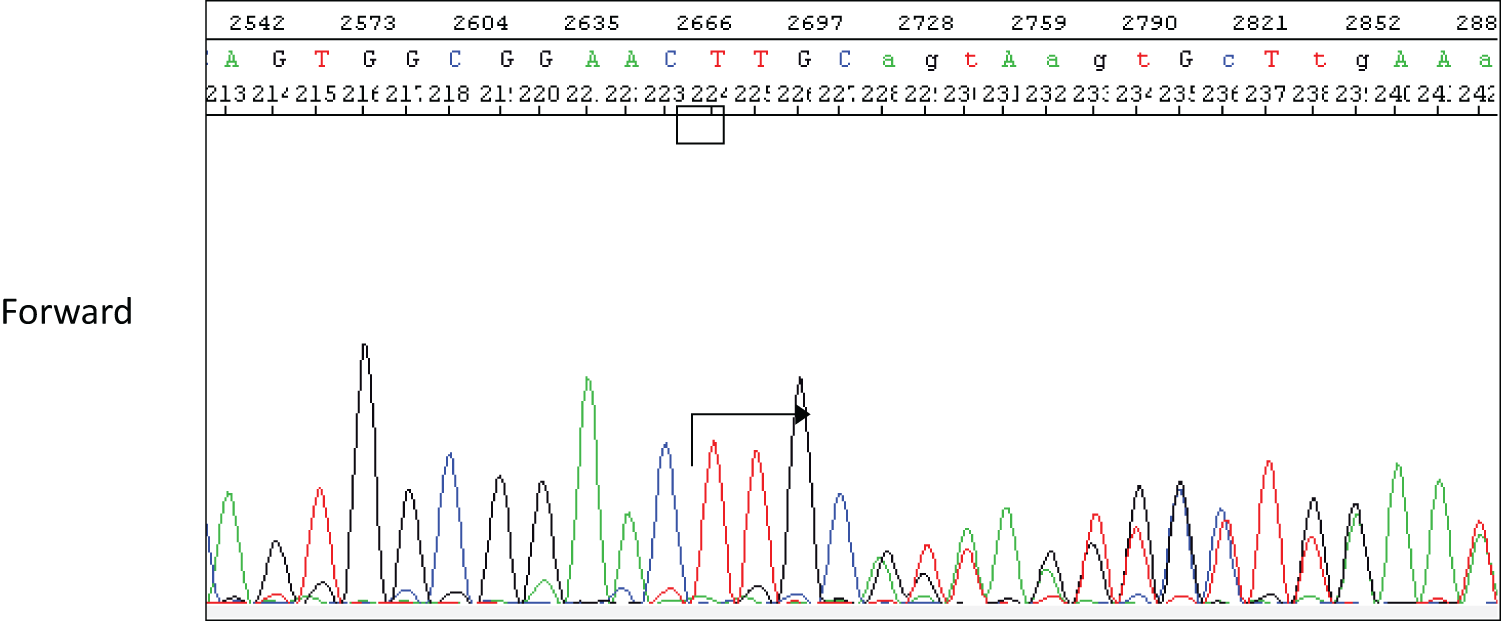
A 44-year-old woman was referred to our hospital because of a right breast tumor identified during a medical checkup. Mammography revealed no tumor or calcification in either breast. However, ultrasonography revealed an irregular hypoechoic mass (22 × 19 × 9 mm) in the right breast (Fig. 1). Dynamic contrast-enhanced magnetic resonance imaging showed an enhanced, irregular mass in the right breast (Fig. 2). Fluorodeoxyglucose positron emission tomography revealed abnormal resorption in the right breast and uterus (Fig. 3). Abnormal resorption in the uterus was diagnosed as a uterine myoma on computed tomography. A core needle biopsy revealed an invasive ductal carcinoma that was estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal receptor 2 (HER2) negative. She had a medical history of bilateral adenomatous goiters for 10 years. Cobblestone papillomatosis was present in the gingiva (Fig. 4). Her mother had been diagnosed with breast cancer, thyroid and tongue tumors, gastric polyps, hepatic hemangioma, and collagen disease. Additionally, her maternal grandmother had been diagnosed with colon cancer. Owing to her family history and physical findings, CS was suspected. The diagnosis of CS was based on the criteria of the National Comprehensive Cancer Network (NCCN).3) In this case, breast cancer and oral papilloma met the major criteria, and thyroid structural lesions met the minor criteria; however, the diagnostic criteria for CS were not met. Despite this, due to the strong suspicion of CS based on her mother’s medical history, genetic testing was performed prior to surgery. From an ethical perspective, professional genetic counseling was provided to the patient and her family, and informed consent for genetic testing was obtained. A direct DNA sequencing analysis was performed, and a haplotype c.634del mutation in exon 7 of the PTEN gene was detected (Fig. 5). This mutation resulted in a p.Asn212Ilefs*9 substitution. Based on the results of the genetic test, she was diagnosed with PHTS.




Although there is no clear evidence for risk-reducing surgery in PHTS, a nipple-sparing mastectomy, sentinel lymph node biopsy, and tissue expander reconstruction were performed. Histopathological examination confirmed an invasive ductal carcinoma measuring 1.8 × 1.0 cm with no lymph node metastasis (Fig. 6). The histological grade was II. Immunohistochemistry revealed ER- and PR-positive staining, HER-2-negative status, and Ki-67-positive staining of 15%. Aromatase inhibitor therapy was started as adjuvant therapy. Five months after breast cancer surgery, the patient underwent a laparoscopic total hysterectomy and bilateral salpingo-oophorectomy, and the histopathological diagnosis was atypical endometrial hyperplasia.

The PTEN gene has two main domains: the N-terminal region, which includes the phosphatase domain, containing the PTEN active site in exon 5, and the C-terminal region, which includes the C2 domain, that binds to phospholipids and positions the catalytic domain on the membrane.4) According to a study investigating cancer risk and genotype–phenotype correlations in 22 Japanese patients with CS, 12 had N-terminal-PTEN variants (11 in the phosphatase domain) and 10 had C-terminal-PTEN variants (C2 domain). Cancer was found in 33.3% (4/12) of the patients in the N-terminal region group (27.3% [3/11] in the phosphatase domain) and 80.0% (8/10) of those in the C2 domain group. The incidence of cancer in the C2 domain group was significantly higher than that in the N-terminal region (p = 0.038) and phosphatase domain (p = 0.023) groups. Breast cancer was found in 100% (7/7) of female patients in the C2 domain group and 50.0% (4/8) of those in the N-terminal group (42.9% [3/7] of those in the phosphatase domain group). The incidence of breast cancer in the C2 domain group was marginally higher than that in the N-terminal region group (p = 0.051).5)
Pathogenic variants of the PTEN gene may include large deletions, small intragenic deletions/insertions and missense, nonsense, and splice site variants, and these have been described in all nine exons of the gene.2,6–8) Unlike other genes, virtually all germline PTEN missense mutations in the coding region are believed to be pathogenic.6,9) Furthermore, no clear genotype–phenotype correlation has been found in PHTS, although several studies have examined the correlation between CS phenotypes and PTEN gene variant sites.2,4,10,11)
The c.634del mutation in the C2 domain has not previously been reported in PHTS. Germline PTEN-related breast cancer is characterized by a high incidence of hormone receptor-positive cases, the progression tends to be slow, and the prognosis is not poor.5)
On the other hand, somatic PTEN mutation has been reported to be associated with triple-negative breast cancers.12) The literature describing both PTEN variants and breast cancer subtypes is presented in Table 1. Hormone receptor-positive cases were the most common, including the present case (8 out of 12 cases), whereas 3 and 1 cases were triple negative and HER2 positive, respectively.13–22)
| Age | Sex | ER | PgR | HER2 | Pathology | Bilateral | Exon | Nucleotide change | Variant type | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 32 | F | + | + | – | IDC | + | 1 | c.71A>T | Missense | Pradella et al., 201413) |
| 33 | F | + | + | – | IDC | + | 5 | p.R130*(c.388C>T) | Nonsense | Nara et al., 201714) |
| 29 | F | + | NA | – | IDC | + | 5 | p.R130Q(c.389G>A) | Missense | Chandhanayingyong et al., 201515) |
| 57 | F | – | – | – | Mucinous | – | 7 | c.697C>T | Nonsense | Gosein et al., 201616) |
| 34 | F | + | NA | – | IDC | – | 7 | c.698_701delGACCinsAA | NA | Walsh et al., 201117) |
| 29 | F | – | – | – | IDC | + | 7 | c.723dupT | Nonsense | Won et al., 201918) |
| 35 | F | + | + | – | DCIS | – | 7 | c.723dupT | Nonsense | Nara et al., 201714) |
| 41 | M | + | NA | NA | IDC | – | 7 | c.802delG | Splicing | Fackenthal et al., 200119) |
| 35 | F | – | – | – | IDC | – | 8 | c.823_840del.18 | Nonsense | Sueta et al., 202220) |
| 44 | F | + | + | – | IDC | + | 9 | c.1027-2A>G | Splicing | Peiró et al., 201021) |
| 38 | F | – | – | + | IDC | – | NA | Mutation | NA | Sabaté et al., 200622) |
| 44 | F | + | + | – | IDC | – | 7 | c.634del | Frameshift | This case |
NA, not assessed; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ.
PHTS is a rare autosomal dominant hereditary disorder caused by germline variants of the PTEN gene located on chromosome 10q23.6,23,24) PTEN participates in the negative regulation of the phosphoinositide 3-kinase-protein kinase B and mammalian target of rapamycin signaling pathways, controlling cell proliferation and cell cycle progression and promoting apoptosis.25) Thus, the loss-of-function mutation of PTEN correlates with the development of various human cancers. In recent studies, it has been found that target the cellular alterations caused by PTEN pathogenic variants.26) Pharmacologic mTOR inhibitors (like everolimus and sirolimus) are being studied for their potential to reduce oncologic risk. Additionally, there is a hypothesis that poly (ADP-ribose) polymerase (PARP) inhibitors may also be effective for PTEN pathogenic variant carriers, similar to their use in BRCA-related breast cancers. However, more research is needed to effectively translate these strategies into prevention and treatment options. The cumulative lifetime risk of any cancer in patients with CS is 89%, with morbidities including 85% for breast cancer, 32% for LDD, 21% for thyroid cancer, 19% for endometrial cancer, 16% for colorectal cancer, and 15% for renal cancer.2,27) Nevertheless, the most typical features are specific mucocutaneous lesions, including trichilemmomas, acral keratoses, and oral papillomatous papules, which occur in 90%–100% of patients with CS.
Cancer surveillance should be performed for individuals with positive findings on genetic testing and those with negative findings on genetic testing but meeting the clinical diagnostic criteria. The NCCN guideline recommends breast self-examination beginning at the age of 18 years, annual clinical breast examinations starting at the age of 25 years, and mammography and breast magnetic resonance imaging (MRI) at 30–35 years of age (or 5–10 years before a family’s earliest known breast cancer diagnosis).28) Given the 85% risk of developing breast cancer, the possibility of risk-reducing mastectomy should be considered. Counseling should include discussions on the degree of protection, reconstruction options, and associated risks. While options like nipple-sparing mastectomy show promise based on BRCA data, the effectiveness of chemoprevention in PTEN pathogenic variant carriers remains unclear. Overall, further research is essential to improve outcomes and clarify treatment strategies for this population.26) Early detection and surveillance of PTEN-related hereditary cancers is done with established imaging modalities such as ultrasound and MRI (Table 2). In the present case, the patient should undergo future surveillance of the left breast, thyroid, kidneys, and colon including annual MRI and mammography for breast evaluation, annual ultrasound for thyroid assessment, ultrasound every 1–2 years for renal cancer surveillance, and a colonoscopy every 5 years for colorectal cancer screening.
| Surveillance | Interval | From age | |
|---|---|---|---|
| Breast cancer | Breast self-examination | Every 1 year | 18 |
| MRI | Every 1 year | 30 | |
| Mammography | Every 1 year | 30–35 | |
| Risk-reducing surgery offered | – | – | |
| Thyroid cancer | Ultrasound | Every 1 year | Age at diagnosis |
| Renal cancer | Ultrasound | Every 1–2 years | 40 |
| Colorectal cancer | Baseline colonoscopy | Every 5 years | 35–40 |
| Melanoma | Baseline skin examination | – | 30 |
| Endometrial cancer | The presence or absence of abnormal bleeding/endometrial biopsy※ | – | – |
※This should include endometrial biopsy every 1–2 years if abnormal uterine bleeding or postmenopausal bleeding occurs.
Herein, we report a case of breast cancer with a newly diagnosed c.634del mutation in the PTEN gene. Because the patient was diagnosed with PHTS before breast cancer surgery, we were able to discuss the breast surgery procedure resulting in her undergoing a right nipple-sparing mastectomy. We also reviewed the current literature on PTEN genetic variants and breast cancer subtypes.
The authors thank all those who contributed to this report.
This study did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.
Authors’ contributionsYM and HJ performed the surgical procedures.
All the authors have read and approved the final version of the manuscript.
Availability of data and materialsThe data are not available for public access due to patient privacy concerns but are available from the corresponding author upon reasonable request.
Ethics approval and consent to participateEthics approval: All procedures involving human participants were performed in accordance with the ethical standards of the Institutional Review Board of Teikyo University Hospital and the 1964 Helsinki Declaration and its later amendments.
Consent for publicationInformed consent was obtained from the patient for the publication of this report.
Competing interestsThe authors declare that they have no competing interests.