2025 年 11 巻 1 号 論文ID: cr.24-0137
2025 年 11 巻 1 号 論文ID: cr.24-0137
INTRODUCTION: In recent years, new molecularly defined tumor groups have been reported among tumors previously considered unclassifiable. Among them, gene fusions involving the CREB family of transcription factors, including cAMP-responsive element modulator (CREM), with genes encoding FET family RNA-binding proteins, such as Ewing sarcoma breakpoint region 1 (EWSR1), have recently been shown to be implicated in driving the pathogenesis of various tumor types. Here, we report our experience with a gastric mesenchymal tumor with epithelioid histology and an EWSR1::CREM fusion, which is rare but requires caution.
CASE PRESENTATION: A 58-year-old man with epigastric pain underwent esophagogastroduodenoscopy, which revealed a submucosal tumor, 40 × 30 mm in size, at the greater curvature of the upper gastric body. Surgical resection was scheduled because of easy bleeding from the tumor and because biopsy could not establish a diagnosis. The tumor was clinically considered benign because there was no significant accumulation on positron emission tomography scans. Therefore, we performed a local resection of the stomach. Histologically, the tumor consisted of a proliferation of keratin-positive, relatively uniform epithelioid cells arranged in sheets, with a scattering of lymphoid follicles in the surrounding area. Based on a pathology consultation, the tumor was diagnosed as a mesenchymal tumor with EWSR1::CREM fusion.
CONCLUSION: We experienced a gastric epithelioid mesenchymal tumor with EWSR1::CREM fusion genes. Since a malignant course has been reported in similar tumors in the stomach and abdominal cavity, such patients require careful follow-up.
angiomatoid fibrous histiocytoma
CCSclear cell sarcoma
CCSLGTclear cell sarcoma-like tumors of the gastrointestinal tract
CREMcAMP-responsive element modulator
EWSR1Ewing sarcoma breakpoint region 1
EWSR1/FUS::CREB fusionEWSR1 or FUS and CREB family fusion
FISHfluorescence in situ hybridization
FUSfused in sarcoma
GNETgastrointestinal neuroectodermal tumor
LECSlaparoscopic and endoscopic cooperative surgery
In recent years, the increasing use of genetic testing in routine surgical pathology practice has led to the discovery of fusion-associated neoplasms, and new molecularly defined tumor groups have been reported among tumors previously considered unclassifiable. Neoplasms with fusions between Ewing sarcoma breakpoint region 1 (EWSR1) or fused in sarcoma (FUS) genes and genes encoding the CREB family of transcription factors (ATF1, CREB1, and cAMP-responsive element modulator [CREM]) are one such tumor type. These fusions have been reported to drive the pathogenesis of various tumor types with mesenchymal, neuroectodermal, and epithelial lineages.1)
Most tumors in the gastrointestinal tract with EWSR1/FUS and CREB family fusions (EWSR1/FUS::CREB fusion) are reportedly clear cell sarcoma-like tumors of the gastrointestinal tract (CCSLGT).2,3) Here, we report our experience with a gastric epithelioid mesenchymal tumor with an EWSR1::CREM fusion that was distinct from established entities.
A 58-year-old man with a history of occasional epigastric pain for approximately 6 months underwent a medical checkup at a local hospital. Stomach fluoroscopy revealed a tumor located at the greater curvature of the upper gastric body, for which he was referred to our hospital.
Esophagogastroduodenoscopy showed a tumor 40 × 30 mm in size, with easy bleeding, an uneven shape, and a deep depression in the center (Fig. 1A). Since the entire tumor surface, including the depression, was covered by noncancerous epithelium, it was diagnosed as a gastric submucosal tumor. Hematoxylin–eosin staining of biopsy specimens showed a spindle cell tumor. However, no definitive diagnosis could be made despite using various types of immunostains. Endoscopic ultrasonography showed a uniform isoechoic mass that appeared to be derived from the fourth layer (i.e., the muscularis propria). A contrast computed tomography scan revealed a contrast-enhanced mass in the upper part of the stomach body (Fig. 1B), with neither invasion of the peripheral organs nor distant metastases. Positron emission tomography showed only mild accumulation in the gastric tumor, with no accumulation in other areas.

CT, computed tomography
Based on these results, we considered the possibility of malignancy to be low, but since the tumor bled very easily and could not be definitively diagnosed by biopsy, we decided to perform local gastric resection. Laparoscopic evaluation showed that the serous surface of the tumor was smooth but appeared hyperemic (Fig. 1C). The technique of laparoscopic and endoscopic cooperative surgery (LECS) was used to perform local gastrectomy. Due to the relatively large size of the tumor, the procedure was performed using inverted LECS with Crown methods,4) which were designed to prevent the outflow of gastric contents. After excising the lesion, the open ends of the stomach wound were aligned and closed with a linear stapler. The surgical time was 182 min, and estimated blood loss was below measurable levels. The patient’s postoperative course was uneventful, and he was discharged 7 days after surgery. Currently, 1 and a half years after surgery, he remains alive without recurrence.
Pathological evaluation of the specimen showed that the tumor, measuring 4.5 × 2.7 × 1.6 cm, was slightly shiny and yellowish-white in color (Fig. 2A). Histopathological assessment revealed relatively homogeneous epithelioid cells extending from the mucosal lamina propria to the muscularis propria and slightly to the subserosal layer, with the cells proliferating in sheets with intervening hyalinized stroma (Fig. 2B). Scattered lymphoid follicles were observed at the periphery of the tumor. The tumor cells had relatively uniform nuclei with a round to oval shape and scant eosinophilic cytoplasm (Fig. 2C). The mitotic count was 3/10 high-power fields, and the cell boundaries were indistinct (Fig. 2C). Silver impregnation staining showed the presence of argyrophil fibers between the tumor cells, suggesting that it was a mesenchymal tumor (Fig. 2D). The surgical margin was negative.

H&E, hematoxylin–eosin
The tumor cells were immunohistochemically positive for D2-40 (diffuse), α-smooth muscle actin (diffuse), Bcl-2 (diffuse), AE1/AE3 (focal) (Fig. 2E), and Kit (focal), and negative for DOG1, desmin, S100, CD34, EMA, β-catenin, ALK, CD21, LCA, and CD68. The Ki-67 proliferation index was approximately 7%. Fluorescence in situ hybridization (FISH) assays showed negative evidence of SS18 rearrangement. Gastrointestinal stromal tumor, solitary fibrous tumor, smooth muscle tumor, desmoid fibromatosis, inflammatory myofibroblastic tumor, follicular dendritic cell sarcoma, and synovial sarcoma were included in the differential diagnosis, but the findings were not typical for any of these diseases. Subsequently, at a pathology consultation, the tumor was found to be negative for MUC4 and SSX (C-term), while FISH analysis detected rearrangements for both EWSR1 and CREM genes (Fig. 3). Based on these findings, the tumor was diagnosed as an epithelioid mesenchymal tumor with EWSR1::CREM gene fusion.
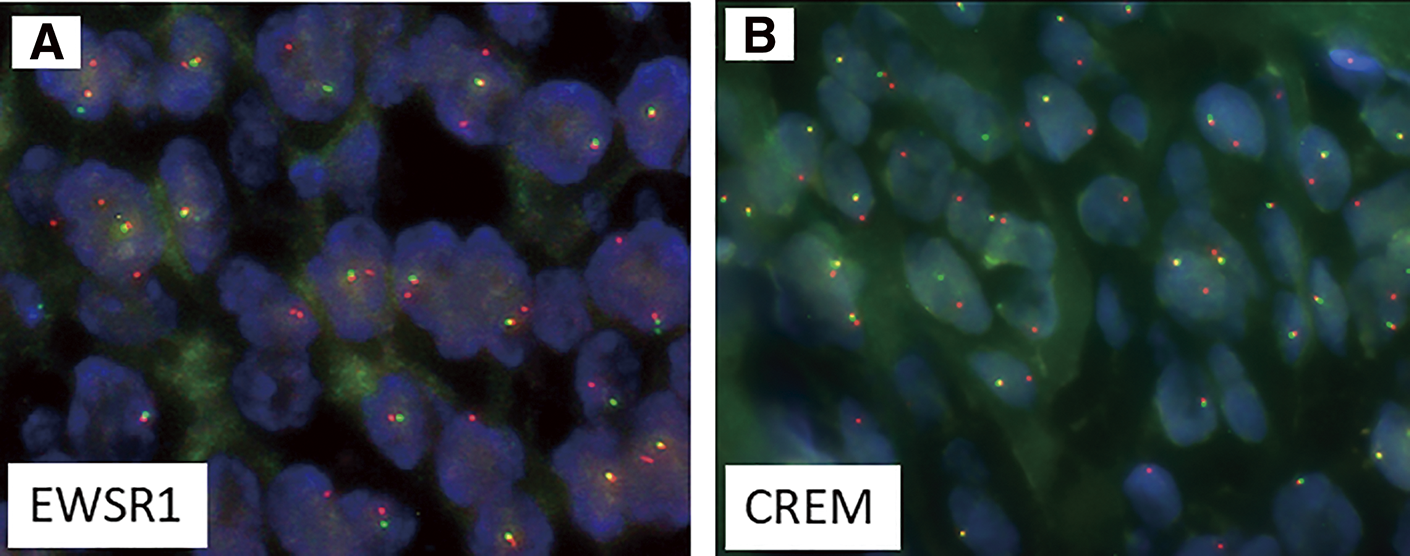
CREM, cAMP-responsive element modulator; EWSR1, Ewing sarcoma breakpoint region 1; FISH, fluorescence in situ hybridization
EWSR1::CREM fusion is one of the fusions between EWSR1 or FUS and the CREB family of genes that have been reported in various tumor entities since approximately 2017.5) Types of tumors with these fusions include angiomatoid fibrous histiocytoma (AFH), soft tissue and gastrointestinal clear cell sarcoma (CCS), primary pulmonary myxoid sarcoma, hyalinizing clear cell carcinoma of the salivary gland, and malignant mesotheliomas.1) EWSR1, along with FUS, belongs to the FET family of RNA-binding proteins.6) EWSR1 is involved in the crucial regulation of proper centromere function and inheritance in interphase cells.7) Meanwhile, CREM, together with ATF1 and CREB1, belongs to the group of transcription factors called CREB family transcription factors.1,2) Among these transcription factors, CREM is a newly recognized member in human tumors, and the phenotypic spectrum associated with CREM fusion is still being clarified.2)
Most gastric tumors with EWSR1/FUS::CREB fusions have been reported to be CCSLGT, also known as gastrointestinal neuroectodermal tumors (GNETs). The tumor in our patient had histological findings distinct from GNETs but was similar to 3 previously reported tumors,1,8,9) which are summarized in Table 1. The 4 patients, including the present case, ranged in age from 25 to 64 years and consisted of 3 men and 1 woman. Although interpreted as mesenchymal tumors, cytokeratin or EMA positivity was observed in 3 of the cases, including our patient. In case 3, which showed a mixture of epithelial and mesenchymal components in gastritis cystica profunda, the disease course was benign, while the other 2 patients experienced metastasis and recurrence. Among these 2 cases, liver metastasis occurred 1 year after diagnosis in the first case, although the patient was subsequently lost to follow-up. The second case underwent resection of the initial gastric lesion and a recurrent lesion, followed by treatment with imatinib, sunitinib, and radiation therapy for the recurrence.
| Case | Age | Sex | Primary site | Size (cm) |
Metastasis | IHC-positive Epithelial markers |
Fusion | Outcome (mo) |
References | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | M | Intra-abdominal continuing from fornix of the stomach |
7 | + | Liver | EMA | EWSR1::CREM | Rec (12) AWD (12) |
1) |
| 2 | 32 | M | Stomach | 3 | + | Liver Peritoneum Lung Spleen |
AE1/AE3 | EWSR1::CREB1 | Rec (48, 56) AWD (122) |
8) |
| 3 | 64 | F | Stomach | 8 | − | EWSR1::CREM | NED (28) | 9) | ||
| Our case | 58 | M | Stomach | 4.5 | − | AE1/AE3 | EWSR1::CREM | NED (20) |
AWD, alive with disease; CREM, cAMP-responsive element modulator; EWSR1, Ewing sarcoma breakpoint region 1; EWSR1/FUS::CREB fusion, EWSR1 or FUS and CREB family fusion; F, female; IHC, immunohistochemistry; M, male; mo, months; NED, alive with no evidence of disease; rec, recurrence.
In a broader context, similar epithelioid mesenchymal tumors with keratin expression and EWSR1/FUS::CREB fusion have been reported in the abdominal cavity outside the stomach, with many of these tumors showing malignant behavior, including recurrence, metastasis, and resistance to chemotherapy.1,8) Considering these previous tumor reports, we believe that our case should be carefully followed up for potential malignancy. There are still very few reports of gastric epithelioid mesenchymal tumors with EWSR1/FUS::CREB fusion that do not fit the criteria for GNETs, and the collection of additional cases is needed to better understand their epidemiology, symptoms, prognosis, and treatment.
We experienced a case of gastric epithelioid mesenchymal tumor with EWSR1::CREM fusion genes that did not fit the diagnostic criteria of established tumor entities. Since a malignant course has been previously reported in the literature for similar tumors, careful follow-up is required for these cases.
The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing.
This study received no specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’contributionsNY drafted the manuscript.
All other authors critically reviewed the manuscript.
NY, HY, and KU performed operations.
KU performed endoscopy and was responsible for preoperative diagnosis.
TY and AY carried out pathological diagnosis.
All authors approved the final manuscript and agreed to take responsibility for all aspects of the study.
Availability of data and materialsData presented in this case report are available from the corresponding author upon reasonable request.
Ethics approval and consent to participateThis work does not require ethical considerations or approval.
Consent for publicationWritten informed consent was obtained from the patient for the publication of this case report and accompanying images.
Competing interestsThe authors declare that they have no competing interests.