2022 Volume 25 Issue 1 Pages 29-40
2022 Volume 25 Issue 1 Pages 29-40
A woman in her seventies presented with leukocytosis. Her white cell count was 33.87 × 103/μL with 80.5% leukemia cells. Her bone marrow was replaced with peroxidase-negative leukemia blasts that were CD10+/−, CD19+, CD20−, CD13+, CD33−/dim, CD34+, CD117−/dim, CD66c+/−, cytoplasmic (cy-) CD79a+, terminal doxynucleotidyl transferase+, and cy-IGHM−. G-banding revealed t(9;22)(q34;q11.2) as the sole chromosome abnormality, and reverse transcriptase polymerase chain reaction (PCR) and nucleotide sequencing confirmed that the fusion encompassed exon 19 of BCR and exon 2 of ABL1, which generate p230 micro (μ)-BCR-ABL1 mRNA. We treated her with a second-generation tyrosine kinase inhibitor, dasatinib, in combination with low-intensity chemotherapy (vincristine and dexamethasone) followed by consolidation, leading to a hematological complete response (CR) and 10−4 level reduction of leukemia cell burden as measured by LightCycler®-based real-time quantitative PCR assay. The patient received maintenance treatment with dasatinib followed by ponatinib and achieved hematological CR for 2 years and 8 months. This report showed that patients with the p230 μ-BCR-ABL1 fusion gene may present with not only chronic myeloid leukemia with mild clinical features but also Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) with precursor B-cell immunophenotype; moreover, dasatinib-based induction, consolidation, and maintenance therapies are as effective for Ph+ ALL with μ-BCR-ABL1 as those with minor- and major-BCR-ABL1.
症例は70代女性.白血球増多のため紹介受診した.白血球数は33.87 × 103/μLで,白血病細胞が80.5%を占めた.骨髄はペルオキシダーゼ陰性の芽球で置換され,それらはCD10+/−, CD19+, CD20−, CD13+, CD33−/dim, CD34+, CD117−/dim, CD66c+/−, 細胞質(cy-) CD79a+, TdT+, cy-IGHM−であった.Gバンディング核型は46,XX,t(9;22)(q34;q11.2)で,付加的異常は認めなかった.Reverse transcriptase polymerase chain reaction (PCR)とシークエンシングの結果,融合遺伝子はBCRのexon 19とABL1のexon 2が接合し,p230 micro (μ)-BCR-ABL1をコードしていた. 第2世代のチロシンキナーゼ阻害剤であるダサチニブと低強度の化学療法(ビンクリスチンおよびデキサメタゾン)を組み合わせた寛解導入療法に続いて強化療法を実施したところ,血液学的な完全奏効(CR)に至った.LightCycler®を基盤としたリアルタイム定量的PCRを構築し寛解状態を評価したところ,白血病細胞は10−4レベルに減少していた.ダサチニブに続いてポナチニブによる維持療法を継続し,2年8か月間血液学的CRを維持している.p230 μ-BCR-ABL1は慢性骨髄性白血病にまれに認められ,やや緩徐な臨床経過をとるとされているが,前駆B細胞の免疫形質を示すフィラデルフィア染色体陽性急性リンパ芽球性白血病(Ph+ ALL)にも認められることが明らかになった.ダサチニブを基本薬とした寛解導入療法・強化療法・維持療法は,p190 minor-/p210 major-BCR-ABL1 Ph+ ALLと同様に,p230 μ-BCR-ABL1 Ph+ ALLにも有効である.
Philadelphia chromosome (Ph) is generated as the result of a reciprocal translocation, t(9;22)(q34;q11.2), which leads to fusion between the 5' part of BCR, normally located on chromosome 22 band q11.2, and the 3' part of ABL1 on chromosome 9 band q34. 1, 2 Ph chromosome and BCR-ABL1 fusion gene are detected in almost all cases of chronic myeloid leukemia (CML) and a fraction of precursor B-cell acute lymphoblastic leukemia (ALL), 1 comprising Ph-positive (Ph+) leukemias.
There are three breakpoint cluster regions (bcrs) within BCR, when the gene is rearranged with ABL1. 1-4 The major (M)-bcr generates the p210BCR-ABL1 chimeric protein, which is found in the majority of CML and one-third of Ph+ ALL patients. The upstream minor (m)-bcr, which generates the p190BCR-ABL1 protein, is primarily associated with Ph+ ALL, but is found in 1% of CML cases. 5 The third breakpoint cluster, micro (μ)-bcr, located downstream of M-bcr, leads to the generation of mRNA encompassing exon 19 (e19) of BCR and exon 2 (a2) (on rare occasions exon 1a) of ABL1, thereby encoding the p230BCR-ABL1 protein. 1-4,6 The p230 μ-BCR-ABL1 fusion gene is observed in a small fraction of CML, characterized by moderate leukocytosis with or without marked thrombocytosis and absence of splenomegaly. 2,7,8 These associations between the breakpoints and particular clinical features have been attributed to the differential transforming capabilities of each BCR-ABL1 chimeric protein. 3,4,9,10
Herein, we report a rare case of a patient with Ph+ ALL carrying the p230 μ-BCR-ABL1 fusion gene. The patient was treated with a second-generation tyrosine kinase inhibitor (TKI), dasatinib, in combination with low-intensity chemotherapy, leading to a favorable hematological and molecular response.
Mononuclear cells were prepared from bone marrow (BM) aspirates using Lymphocyte Separation Solution (Nacalai Tesque, Kyoto, Japan) and subjected to flowcytometry (FCM). Intracellular antigens were analyzed after Dako’s IntraStain® treatment (Agilent, Santa Clara, CA). Fluorescence was captured using a NAVIOS 3L flow cytometer and analyzed by Kaluza Flow Cytometry Analysis Software (Beckman Coulter, Brea, CA).
G-banding and fluorescence in situ hybridizationCytogenetic preparations were obtained according to the established method and chromosomes were banded by trypsin-Giemsa. Two fluorescence in situ hybridization (FISH) probes, the Vysis LSI BCR/ABL dual color, dual fusion translocation probe (DF probe) and the Vysis LSI BCR/ABL ES dual color translocation probe (ES probe), were purchased from Abbott Laboratories (Abbott Park, IL). Denaturing of the chromosome/probe, hybridization, and washing conditions were as recommended by the manufacturer. FISH results were analyzed using a fluorescence microscope (Nikon Corporation, Tokyo, Japan) equipped with DAPI, fluorescein isothiocyanate (FITC), and tetramethylrhodamine B isothiocyanate (TRITC) fluorescence filters, as well as a DAPI/FITC/TRITC triple bandpass filter (Nikon Corporation).
Reverse transcriptase polymerase chain reaction and direct sequencingTotal cellular RNA was prepared with an RNeasy Mini Kit (QIAGEN, Hilden, Germany). First-strand cDNA was synthesized from 2 μg of total RNA in a reaction mixture containing random hexamer primers (Roche Applied Science, Penzberg, Germany) and SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA). The sequences of primers for reverse transcriptase polymerase chain reaction (RT-PCR) are listed in Supplementary Table S1. 8 PCR amplification was performed in a Veriti 96-well thermal cycler (Thermo Fisher Scientific, Waltham, MA). The PCR products were visualized by ethidium bromide-stained agarose gel electrophoresis, excised from the gel, purified using a NucleoSpin Gel and PCR clean-up kit (Takara Bio, Otsu, Shiga, Japan), subjected to the cycle sequencing reaction (BigDye® Direct Cycle Sequencing Kit; Thermo Fisher Scientific), and then sequenced using a SeqStudioTM Genetic Analyzer (Thermo Fisher Scientific).
LightCycler® platform-based real-time quantitative PCR assayFITC-labeled 5′ and LightCycler® Red640-labeled 3′ hybridization probes were designed by LightCycler® Probe Design Software 2.0 (Roche Applied Science; Supplementary Table S1 and Supplementary Figure S1A). The fluorescence signal was plotted against the cycle number for 100 to 108 serial dilutions of the 130-bp e19a2 BCR-ABL1 fragment cloned into the TArget ConeTM plasmids (TOYOBO, Osaka, Japan), and a standard curve was constructed by plotting the crossover point against the log10 of the copy number of target DNA (Supplementary Figure S1B). The number of target molecules in clinical materials of interest was then calculated automatically by reference to this curve as the copy number of p230 μ-BCR-ABL1 mRNA per μg of total RNA. Finally, normalized levels were calculated as the ratios between copy numbers of p230 μ-BCR-ABL1 and ABL1. 11
A woman in her seventies was referred to the hematology department due to leukocytosis, which was incidentally detected by a blood test for regular follow-up of cecum carcinoma after ileocecectomy conducted 5 years earlier. One week after the initial presentation, her hemoglobin level was 12.7 g/dL, white cell count increased from 11.94 × 103/μL to 33.87 × 103/μL during the week, and platelet count was 118 × 103/μL. The white cell differential was 3.0% lymphocytes, 2.0% monocytes, 4.0% eosinophils, 0.5% basophils, 8.5% segmented neutrophils, 1.5% banded neutrophils, and 80.5% leukemia cells. Lactate dehydrogenase was 400 U/L and uric acid was 6.3 mg/dL.
BM was hypercellular and contained 79.5% leukemia blasts negative for peroxidase staining (Figure 1a to d). FCM revealed that the cells were CD10+/−, CD19+, CD20−, CD21−/dim, CD22−/dim, CD24+/−, CD25+/−, CD13+, CD33−/dim, CD34+, CD117−/dim, CD38+, CD45RA+, CD66c+/−, and HLA-DR+ (Figure 2A). Intracellular antigens were cytoplasmic (cy-) CD3−, cy-CD79a+, terminal doxynucleotidyl transferase (TdT)+, myeloperoxidase−, and cy-IGHM− (Figure 2B). Examination of the biopsy specimens confirmed replacement of the marrow space with immature blasts, which were positive for TdT and CD79a (Figure 1e to g), and negative for CD20 and CD3.

Appearance of leukemia blasts in BM. (a and b) Wright-stained leukemia cells in aspirate smear preparation, showing small blasts to larger cells with moderate amount of light-blue cytoplasm (original magnification, 100× objective lens). (c) Peroxidase (POX) staining, showing POX-negative blasts and POX-positive myeloid progenitors (100×). (d) Hematoxylin & eosin (H&E) staining of clot section, showing proliferation of leukemia blasts with scattering eosinophils (40×). (e) H&E-stained biopsy specimen, showing replacement of the marrow space with blasts (20×). The blasts show cytoplasmic positivity for CD79a (f, 100×) and nuclear positivity for TdT (g, 100×)

FCM of leukemia blasts in BM. (A) FCM for cell surface antigen expression. Gated cells on the SSC/FSC scattergram are subjected to a single-color analysis. Positive cell populations for each antigen are colored green. Their percentages are indicated above the horizontal bars. (B) FCM detecting intracellular antigens. “c” denotes cytoplasmic. MPO, myeloperoxidase.
The karyotype determined by G-banding of the metaphase cells from the BM was 46,XX,t(9;22)(q34;q11.2)[6]/46,XX[4] (Figure 3A). FISH applied to the BM smear slides showed that the leukemia cells nuclei were labeled by one red, one green, and two fusion (yellow) signals (1R1G2Y) after the DF probe, and two red, one green, and one fusion signals (2R1G1Y) after the ES probe (Figure 3B), excluding the m-bcr breakpoint.

Cytogenetic analysis. (A) G-banding karyotype obtained from short-term culture of BM cells. t(9;22)(q32;q11.2) is indicated by the arrows. No additional abnormalities are found. (B) FISH of interphase nuclei using the BCR-ABL1 dual-color DF and ES probes. Pictures through DAPI, rhodamine, FITC, and triple-band pass filters are aligned. Hybridization signals are indicated by arrowheads of their respective colors. Diagrams of the two probes provided by the manufacturers are shown at the bottom of each hybridization series.
RT-PCR using the m-bcr–type primer combination detected m-BCR-ABL1 transcripts after the second-round amplification of the nested PCR (data not shown). The M-bcr–type primer combination generated PCR products larger than those of the M-BCR-ABL1 e13a2 (formerly b2a2; 455 bp) fusion gene, and the larger-size products became more evident by the second round of PCR using the inner primer combination (Figure 4A). 8 Next, we applied the bcr-c-e19 forward and abl-e2 reverse primer combination and obtained 137-bp products that matched the calculated size of the μ-BCR-ABL1 fusion mRNA (Figure 4B). Finally, we performed direct sequencing of the PCR products and confirmed the e19a2 mRNA junction (Figure 4C).

RT-PCR and sequencing of p230 μ-BCR-ABL1 mRNA. (A) First and second round of nested RT-PCR for the M-bcr–type transcripts. The 100-bp ladder as a molecular weight marker (M); CML cells with the p210 e13a2 (formerly b2a2) M-BCR-ABL1 as a control; and BM cells. The PCR products were run through an ethidium-bromide-stained agarose gel. (B) RT-PCR for the μ-bcr-type transcripts using primers for BCR exon 19 and ABL1 exon 2. (C) Nucleotide sequence of the PCR products encompassing the e19a2 μ-BCR-ABL1 mRNA junction. Designations and sequences of PCR primers are described in Supplementary Table S1. Fwd, forward; Rev, reverse.
The international scale-standardized M-BCR-ABL1 mRNA level was 1.2189% and the level of m-BCR-ABL1 mRNA was 1.9 × 102 copies per μg of RNA with reference to the level of GAPDH. The BCR-ABL1 kinase domain mutation screening test detected a deletion mutation, c.550_822del273/p.L184_K274del (COSMIC ID, 4966112), that lead to a complete loss of ABL1 exon 4; however, it is not considered oncogenic and is catalytically inactive. 12 These data were obtained from outside laboratories.
Treatment courseThe patient was informed and consented to the treatment using the European Working Group on Adult ALL study number 01 for Ph+ ALL (EWALL-PH-01) protocol, which was developed for older patients with Ph+ ALL (Figure 5). 13 The day after the prophase dose of dexamethasone, the patient developed tumor lysis syndrome associated with disseminated intravascular coagulation, as evidenced by increased levels of fibrin/fibrinogen degradation products (FDPs; peak value, 483.8 μg/mL) and D-dimer (peak value, 167.2 μg/ml), and decreased levels of fibrinogen (bottom value, 41 mg/dL). Although leukemia cells transiently increased during the prophase treatment, the cells promptly disappeared from the blood after the initiation of dasatinib, in association with transient re-increase of FDPs. Four weekly doses of vincristine and dexamethasone were administered as scheduled. 13 BM examination on day 38 of the treatment revealed that the blasts had decreased to 3.2%. Because of the shortage of methotrexate supply at that time, we temporally withheld dasatinib and performed first-cycle consolidation therapy consisting of cytarabine 500 mg/m2 every 12 hours on days 1, 3, and 5 in combination with prophylaxis for central nervous system involvement (intrathecal methotrexate, cytarabine, and prednisone [triple IT]). Intensive supportive treatments were administered to prevent infectious or hemorrhagic complications resulting from severe and prolonged neutropenia and thrombocytopenia. BM blasts became negative with normal hematopoietic recovery on day 101, achieving a hematological complete response (CR) (Figure 5). Then, we performed three additional cycles of consolidation (two cycles of methotrexate 500 mg/m2, avoiding asparaginase; one cycle of four doses of cytarabine 500 mg/m2) in addition to one additional dose of triple IT, according to the fitness and frailty of the patient.
Figure 5 summarizes the assessment of leukemia cells in the BM during the early period of the therapy by microscopy, FISH with the BCR-ABL1 DF probe, and RT-PCR and real-time qPCR targeting the p230 μ-BCR-ABL1 fusion transcripts, demonstrating that, after induction and one cycle of consolidation treatment, test results of the former three methods became negative and the reduction of leukemia cell burden achieved the level of 10−4.
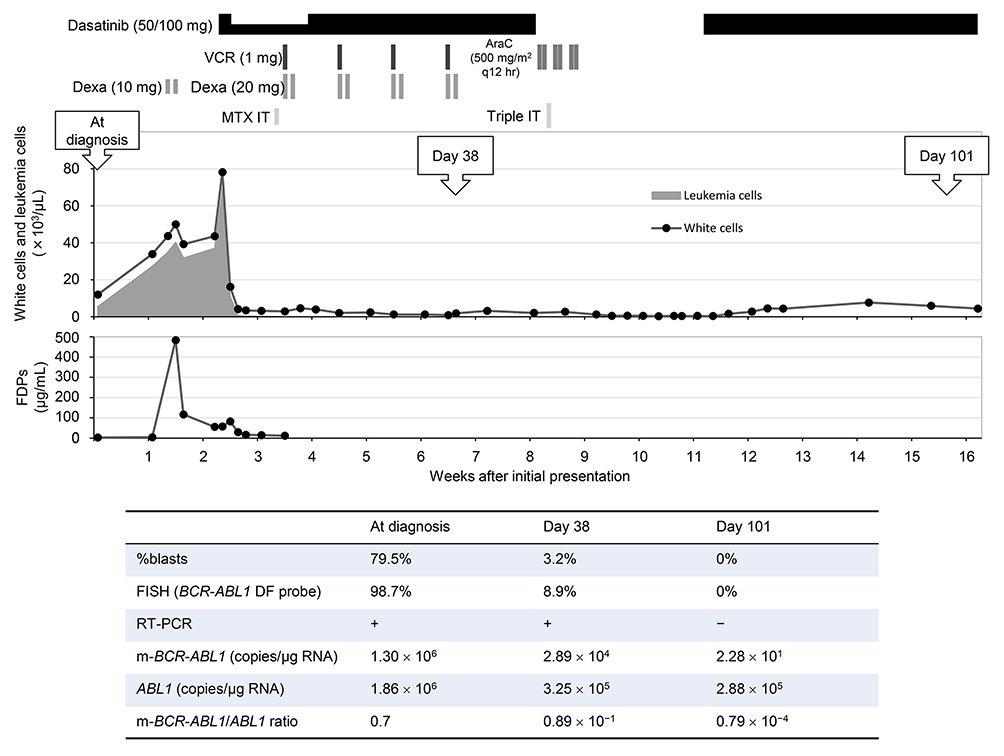
Early course of the treatment. (Top figure) Prophase, induction, and first-cycle consolidation therapy along with white cell/leukemia cell count and levels of fibrin/fibrinogen degradation products (FDPs). VCR, vincristine; AraC, cytarabine; Dexa, dexamethasone; MTX IT, intrathecal methotrexate 15 mg; triple IT, intrathecal methotrexate 15 mg, cytarabine 40 mg, and prednisolone 20 mg. The EWALL-PH-01 protocol for patients > 70 years consists of prophase treatment (Dexa 10 mg/day for 5 days and MTX IT 15 mg), induction treatment (dasatinib 100 mg/day in combination with weekly VCR 1 mg and Dexa 20 mg for 2 days for 4 weeks), and six cycles of consolidation treatment (dasatinib 100 mg/day sequentially with MTX 1,000 mg/m2 on day 1 and asparaginase 10,000 IU/m2 intramuscularly on day 2 for cycles 1, 3, and 5; and AraC 1,000 mg/m2 every 12 hours days 1, 3, and 5 for cycles 2, 4, and 6, with 4-week cycles); prophylaxis for central nervous system involvement includes a total of six injections of triple IT.13 (Bottom table) Evaluation of leukemia cell burden in the BM by morphological examination, FISH, RT-PCR, and real-time qPCR
As the maintenance treatment of the EWALL-PH-01 protocol was considered unfeasible to the patient, we proceeded directly to the post-maintenance therapy consisting of single-agent dasatinib 7 months after the initiation of therapy. 13 The dose of dasatinib was reduced from 100 mg/day to 50 mg/day due to thrombocytopenia.
Two years and 2 months after initial presentation, RT-PCR for p230 μ-BCR-ABL1 mRNA in the peripheral blood became positive. The p230 μ-BCR-ABL1/ABL1 ratio exceeded the level of 10−3 and finally rose to > 10−2 (Figure 6). Although the BM remained in hematological CR under the microscopic assessment, FISH with the BCR-ABL1 DF probe detected nuclei with 1R1G3Y signals, suggesting appearance of double Ph clone. As the disease was considered to acquire resistance to dasatinib, we changed the drug to ponatinib (15 mg/day to 30 mg/day); however, < 10−3 reduction of the ratio was not achieved (Figure 6).
Herein, we described an elderly patient with Ph+ ALL carrying the p230 μ-BCR-ABL1 fusion gene and her treatment course for 3 years. The patient lacked preceding leukocytosis indicative of chronic phase and G-banding revealed no additional cytogenetic abnormalities typical of blast phase, supporting that her disease did not represent lymphoid blast crisis of CML, but de novo occurrence of Ph+ ALL. Detection of low-level M- and m-BCR-ABL1 mRNA was likely to be due to alternative splicing of the fusion gene. The patient was treated with dasatinib-combined low-intensity induction chemotherapy followed by consolidation and maintenance therapies and maintained hematological CR for 2 years and 8 months.
BCR breakpoints are correlated with the phenotypical and clinical features of Ph+ leukemias. 1-4 Several patients with p230 μ-BCR-ABL1 have been described with a myeloproliferative disorder similar to classic CML but with mild clinical symptoms. 7 These include lower peripheral blood leukocyte counts consisting principally of neutrophils, thrombocytosis, less severe splenomegaly, and delayed or absent transformation to blast crisis. 2,7,8 Cases with these characteristics may be called chronic neutrophilic leukemia or neutrophilic CML rather than CML. 4,7,8,14 However, other patients with p230 μ-BCR-ABL1 appear to have typical CML and cases presenting with accelerated/blast phase have been described. 6,15-18 Thus, further studies are needed to determine whether CML patients with p230 μ-BCR-ABL1 indeed comprise a distinct clinical entity within Ph+ leukemias. On the other hand, a literature review found a single case of Ph+ ALL that carried t(9;22)(q34;q11.2) with additional chromosome abnormalities and p230 μ-BCR-ABL1 at presentation, 19 and our report confirmed that patients with the fusion gene may present with Ph+ ALL with precursor B-cell immunophenotype. Nevertheless, as the number of reported cases was quite small; thus, it remains to be determined whether clinical pictures of Ph+ ALL with p230 μ-BCR-ABL1 are similar to or different from those with p190 m-BCR-ABL1 and p210 M-BCR-ABL1.
TKI is currently an essential component of front-line therapy for patients with Ph+ ALL. 20 For elderly patients, addition of imatinib to the consolidation/salvage phase of chemotherapy was associated with a higher rate of CR and superior disease-free and overall survivals with little incremental toxicity compared with historical controls treated with chemotherapy alone. 21 On the other hand, imatinib-based induction led to hematological CR in 26 (96%) of 27 and 29 (100%) of 29 elderly patients with Ph+ ALL. 22,23 This effect of imatinib is strengthened by inclusion of second and third generation TKIs into a variety of therapeutic backbones, 20,24,25 and combined chemotherapy for induction treatment has been de-intensified due to the significant activity of TKI. 13,20,26 The EWALL-PH-01 trial, in which dasatinib substituted for imatinib, included 71 Ph+ ALL patients (54 with m-BCR-ABL1 and 17 with M-MCR-ABL1) aged 55 years or older. 13 The study found that 96% achieved CR and 65% achieved a major molecular response (such as 3-log reduction of BCR-ABL1). 13 At five years, overall and relapse-free survival rates were 36 and 28%, respectively. 13 The current patient aged > 70 was treated with dasatinib (100 mg daily) in combination with vincristine and dexamethasone, and achieved a CR and 4-log reduction of leukemia cell burden after the first cycle consolidation with reduced-dose cytarabine. Thus, this indicates that dasatinib-based induction and consolidation therapies are as effective for elderly Ph+ ALL patients with μ-BCR-ABL1 as those with m- and M-BCR-ABL1. Nevertheless, caution is warranted as tumor lysis syndrome may develop immediately after initiation of therapy.
A common mechanism of TKI resistance in Ph+ leukemia involves point mutations in the kinase domain of BCR-ABL1, which impairs the activity of the available TKIs. 27 In imatinib-treated CML patients with p230 μ-BCR-ABL1, Y253H within the P loop and E355G within the catalytic domain, both of which are sensitive to dasatinib, have been described. 27-29 On the other hand, in the EWALL-PH-01 trial, 36 patients relapsed and 18 of 24 patients analyzed harbored the T315I mutation, 13 which is known as the “gatekeeper” mutation, displaying resistance to all currently available TKIs except ponatinib. 30 In the present case, the emergence of the dasatinib-resistant clone was evident from the consistent increase of p230 μ-BCR-ABL1/ABL1 ratio during the maintenance treatment (Figure 6); thus, we changed dasatinib to ponatinib. Nevertheless, as ponatinib was not as effective as expected, we are considering the use of blinatumomab or inotuzumab ozogamicin when the patient develops florid relapse.

Maintenance treatment with dasatinib followed by ponatinib along with the p230 μ-BCR-ABL1/ABL1 ratios in the blood. RT-PCR for p230 μ-BCR-ABL1 mRNA was constantly positive during this period.
We described here an elderly patient with rare Ph+ ALL carrying the p230 μ-BCR-ABL1 fusion gene. The patient favorably responded to dasatinib-based induction, consolidation, and maintenance treatment. It is suggested that this treatment approach is as effective for elderly Ph+ ALL patients with μ-BCR-ABL1, p190 m-BCR-ABL1, and p210 M-BCR-ABL1.
This work was supported by the Tenri Foundation.
The authors declare that they have no conflict of interest.
All procedures performed in this study involving the patient were conducted in accordance with the 1964 Helsinki Declaration.
The patient consented to the use of her medical record and clinical materials for research purposes.