2022 Volume 25 Issue 1 Pages 48-59
2022 Volume 25 Issue 1 Pages 48-59
A man in his seventies presented with a bulky abdominal tumor. The level of lactate dehydrogenase was 3,854 U/L, uric acid was 11.1 mg/dL, and soluble interleukin-2 receptor was 5,750 U/mL (reference range, 145 to 519 U/mL). The bone marrow (BM) was infiltrated with large cells with features of Burkitt lymphoma, whereas lymphoma cells in the peripheral blood (PB) exhibited indolent cytomorphology indicative of follicular lymphoma (FL). Multicolor flowcytometry and fluorescence in situ hybridization revealed that BM lymphoma cells were CD10+, CD24bright, and CD38bright and had t(14;18)(q32;q21)/BCL2-IGH and t(8;14)(q24;q32)/MYC-IGH. In contrast, the majority of PB lymphoma cells were small to medium sized and CD10−, CD24−/dim, and CD38− and had t(14;18)/BCL2-IGH but lacked t(8;14)/MYC-IGH, and we identified a intermediate population composed of medium-sized cells that were CD10dim, CD24dim/+, and CD38+ and had both t(14;18)/BCL2-IGH and t(8;14)/MYC-IGH. Multiplex polymerase chain reaction confirmed that BM and PB lymphoma cells shared common IGK rearrangement and BCL2-IGH fusion sequence. It is indicated that, in this case, indolent FL and MYC/BCL2 double-hit high-grade B-cell lymphoma (HGBL) transformed from FL developed concurrently and acquisition of t(8;14)/MYC-IGH may not immediately lead to transformation to HGBL, but instead, florid cytomorphologic transformation may occur in BM.
症例は70代男性.腹部の巨大腫瘍のため紹介受診した.LDH 3,854 U/L,尿酸11.1 mg/dL,可溶性インターロイキン2受容体5,750 U/mL(参考値145–519 U/mL)であった.骨髄にはバーキットリンパ腫・白血病細胞に類似した大型の細胞が浸潤していたが,末梢血中のリンパ腫細胞は濾胞性リンパ腫を示唆する低悪性度リンパ腫細胞の形態を示した.マルチカラーフローサイトメトリーとFISHで解析したところ,骨髄中のリンパ腫細胞はCD10+, CD24bright, CD38brightで,t(14;18)(q32;q21)/BCL2-IGH転座とt(8;14)(q24;q32)/MYC-IGH転座が認められた.一方,末梢血中のリンパ腫細胞の多くは小型から中型で,CD10−, CD24−/dim, CD38−の形質を示し,t(14;18)/BCL2-IGH転座陽性,t(8;14)/MYC-IGH転座陰性であった.さらに,中型でCD10dim, CD24dim/+, CD38+を示し,t(14;18)/BCL2-IGH転座とt(8;14)/MYC-IGH転座の両者を有する中間的な細胞集団を末梢血中に見出した.マルチプレックスPCRでは,骨髄と末梢血中のリンパ腫細胞に共通したIGK遺伝子再構成とBCL2-IGH融合遺伝子を認めた.これらの結果から,本症例では低悪性度の濾胞性リンパ腫と濾胞性リンパ腫からトランスフォームしたMYC/BCL2ダブルヒット高悪性度B細胞リンパ腫が同時に発症したと考えられた.さらに,濾胞性リンパ腫細胞がt(8;14)/MYC-IGHを獲得しても直ちに高悪性度リンパ腫にトランスフォームするのではなく,細胞形態の変化を伴うトランスフォーメーションは骨髄で生じることが示唆された.
The WHO Classification of Tumours of Haematopietic and Lymphoid Tissues, published in 2017, proposed the category high-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements.1 The lymphoma has been preferably referred to as double- or triple-hit lymphoma (DHL/THL), and the most frequent double-hit is the combination of a rearrangement of MYC and that of BCL2.2 Morphologically, these cases resemble either diffuse large B-cell lymphoma (DLBCL), not otherwise specified or B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma (BL), and less commonly mimic lymphoblastic lymphoma.1,3-5
Although this lymphoma category is primarily indicated for de novo cases,1 patients often have a history of pre-existing follicular lymphoma (FL) and cases with concurrent occurrence of FL and BL-like histopathology in the same biopsy specimens have been described.3,5-9 We describe here a patient with MYC/BCL2 double-hit HGBL who had no medical history indicative of low-grade lymphoma. The bone marrow (BM) was infiltrated with lymphoma cells with BL-like cytomorphology, whereas circulating cells exhibited that of indolent FL. We present the cytomorphologic, immunophenotypic, and cytogenetic spectrum of lymphoma cells in BM and peripheral blood (PB).
A man in his seventies presented with a bulky abdominal tumor. He had been well until 1 month before presentation, when he became aware of pain throughout the abdomen associated with loss of appetite. From the same time, he noticed night sweats. Computed tomography (CT) scans taken at another hospital revealed a huge tumor within the abdomen, and he was then referred to our department. He lost 7 kilograms of body weight. He had no medical history of low-grade B-cell lymphoma.
On examination, his body temperature was 36.1°C, blood pressure was 105/69 mmHg, pulse was 92 beats/min, and oxygen saturation was 96%. Cervical and axillary lymphadenopathy were unclear, but bilateral inguinal lymph nodes were palpable. A firm tumor was recognized by palpation in the middle of the abdomen. Ascites was not found. His ECOG performance status was 1.
His hemoglobin level was 12.9 g/dL, white cell count was 12.34 × 103/μL, and platelet count was 188 × 103/μL. The level of lactate dehydrogenase was 3,854 U/L, aspartate aminotransferase was 79 U/L, alanine aminotransferase was 22 U/L, total bilirubin was 0.7 mg/dL, total protein was 6.5 g/dL, albumin was 3.2 g/dL, globulin was 3.3 g/dL, creatinine was 1.3 mg/dL, uric acid was 11.1 mg/dL, C-reactive protein was 35.68 mg/dL, and soluble interleukin-2 receptor was 5,750 U/mL (reference range, 145 to 519 U/mL). Fibrin degradation product was 21.5 μg/mL (reference range, ≤ 5 μg/mL), D-dimer was 6.9 μg/mL (reference range, ≤ 1 μg/mL), and soluble fibrin monomer complex was 11.9 μg/mL (reference range, ≤ 7 μg/mL).
The differential of PB white cells was 19.5% lymphocytes, 3.5% atypical lymphocytes, 14.0% monocytes, 0.5% eosinophils, 0.5% basophils, 46.0% segmented neutrophils, 9.5% banded neutrophils, 5.5% metamyelocytes, and 1.0% myelocytes. The cells classified into atypical lymphocytes were small to medium sized and showed high nuclear to cytoplasmic ratio and condensed nuclear chromatin without nucleoli, and a high proportion of the cells had deep and narrow nuclear clefts (Figure 1A). In contrast, BM was infiltrated with large cells with deeply basophilic cytoplasm containing vacuoles, resembling the morphology of BL (Figure 1B). The BM biopsy specimen had 60–70% cellularity with infiltration of medium to large lymphoma cells with indistinct cell borders (Figure 1C). The cells had nuclei with dispersed chromatin and one or two nucleoli. There were many mitotic figures and a few starry-sky macrophages. The cells were positive for CD20 and CD79a and weakly positive for CD10 and BCL2. Nuclear MYC expression was > 70% positive and Ki-67 positivity was > 90% (Figure 1C). CD5, BCL6, cyclin D1, and Epstein-Barr virus–encoded small RNA were negative.
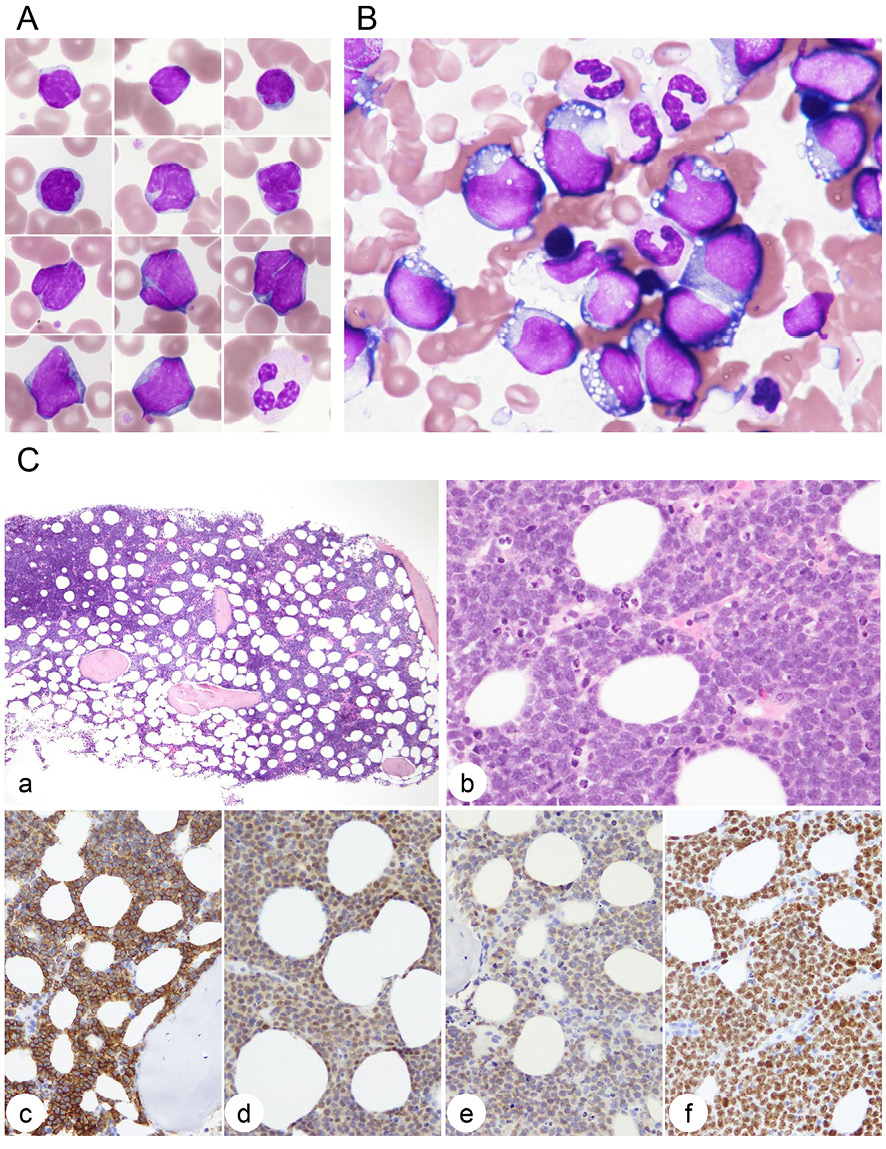
Wright-stained lymphoma cells in PB (A) and BM (B) (original magnification, 100× oil immersion lens). In A, a neutrophil is shown for cell size control (right, bottom). (C) Bone marrow biopsy. a, hematoxylin & eosin (H&E) staining (4× objective lens); b, H&E staining (40×); c, anti-CD20 immunostaining (20×); d, anti-MYC (20×); e, anti-BCL2 (20×); and f, anti-Ki67 (20×)
18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) combined with CT revealed that the tracer was accumulated within the huge abdominal tumor originated from the mesentery and the hilum of the left kidney (Figure 2). There were multiple FDG-avid lymph nodes in the cervical, supraclavicular, axillary, mediastinal, subphrenic, paraaortic, iliac and inguinal regions. Increased tracer uptake was also seen in the clivus, spinal vertebrae, scapula, sternum, pelvis, and proximal portions of humerus and femur bones (Figure 2). The level of splenic uptake was comparable to that of the liver.
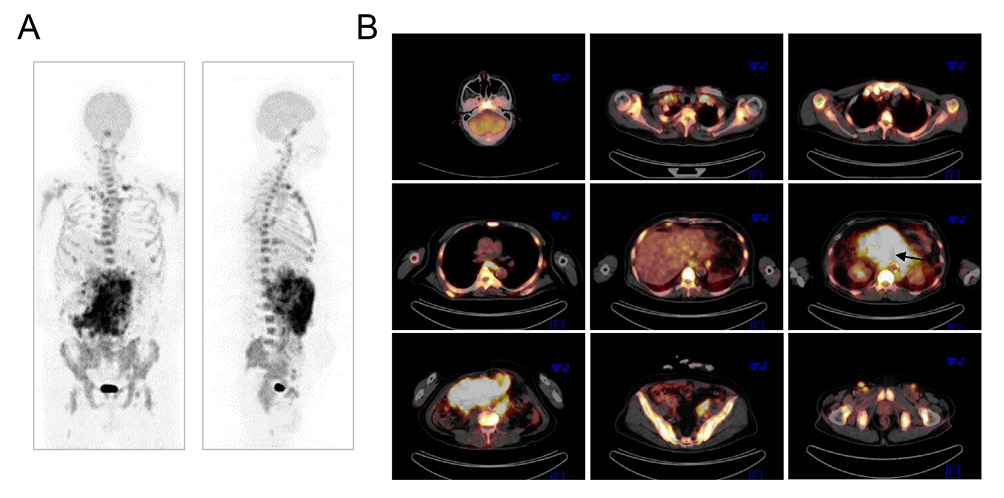
18F-FDG-PET/CT. (A) Anterior and lateral views of the maximum-intensity projection image. (B) Representative fused axial images. The maximum standardized uptake values (SUVmax) of the abdominal tumor and the marrow space of the pelvic bone were calculated as 22.10 and 16.58, respectively. It is noted that the superior mesenteric artery courses through the abdominal tumor (arrow).
The patient was diagnosed with stage IV HGBL and was treated with 6 cycles of dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) followed by two doses of high-dose methotrexate for prophylaxis of central nervous system involvement. 18F-FDG-PET/CT after completion of the therapy confirmed resolution of the FDG-avid lymphoma lesions.
Mononuclear cells of BM and PB materials were selected using the CD45/side scatter (SSC) and/or forward scatter (FSC)/SSC gating strategy, and the cells were subjected to multicolor flow cytometry (FCM). The majority of lymphoma cells in BM were large, as indicated by high FSC intensities, and expressed monoclonal surface immunoglobulin IgG/λ. The cells were CD5−, CD10+, CD19+, CD20+, CD21−, CD22dim, and CD23−, and expression of CD24 and CD38 were bright (Figure 3A). A small fraction of BM lymphoma cells exhibited low FSC intensities and CD10− and CD38− (gate C in Figure 3A). On the other hand, lymphoma cells in PB were divided into two populations: the major population consisted of small to medium-sized cells that were IgG/λ+, CD5−, CD10−, CD19dim, CD20+, CD21−, CD22dim, CD23−, and CD38− (gate C in Figure 3B), whereas cells of the minor population were medium sized and CD10dim and CD38+ (gate D in Figure 3B). DNA indices of both BM and PB lymphoma cells were 1.00 (not shown).

Multicolor FCM of lymphoma cells in BM (A) and PB (B). Large cells in BM, small to medium-sized cells in BM and PB, and medium-sized cells in PB are colored in orange, green, and red, respectively. Expression of CD21, CD22, CD23, and CD24 and that of immunoglobulin heavy chains were assayed separately (bottom). In the FSC/SSC scattergram in A (top, second from left), the pulse parameter settings of FSC were adjusted to effectively separate large cells, so that the position of neutrophil distribution is different between A and B (top, second from left). Thus, green-colored small cell populations in A and B are not exactly the same.
We next divided the CD19dim/+ B-cells in PB into small and medium-sized cells according to FSC intensities at a threshold defined close to the lowest FSC intensity of CD10dim/CD20+/CD38+ cells (Figure 3B; middle, right) and investigated the expression of CD24 in each cell size population. As indicated in Figure 4, the level of CD24 expression in small cells was negative to dim and that in medium-sized cells was dim to positive compared with the level in non-neoplastic B-cells included in the small cell population.
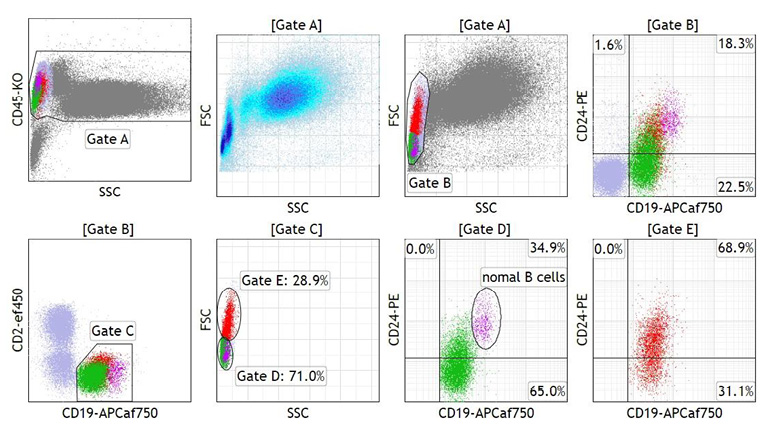
Correlation between the level of CD24 expression and cell size in PB lymphoma cells. Small cells (gate D) and medium-sized cells (gate E) were gated within the FSC/SSC scattergram of CD19dim/+ B-cells (gate C) at the FSC intensity value indicated by the arrow in the middle, right panel in Figure 3B and separated by CD24/CD19 expression. Both small (green) and medium-sized (red) lymphoma cell populations dimly expressed CD19 compared with normal B-cells (purple).
G-banding of metaphase spreads obtained from BM demonstrated t(8;14)(q24;q32) and t(14;18)(q32;q21), and unidentified chromosomal materials were found to be added to the telomeric end of the long arm of chromosome 1 [add(1)(q44)] (Figure 5A). Fluorescence in situ hybridization (FISH) using the BCL2-IGH dual-fusion (DF) and BCL2 break-apart (BA) probes and MYC-IGH DF and MYC BA probes confirmed the t(8;14)(q24;q32) and t(14;18)(q32;q21), generating the MYC-IGH and BCL2-IGH fusion gene, respectively, and add(1)(q44) was labeled with the unrearranged MYC signal (Figure 5B). Accordingly, interphase nuclei hybridized with these probes demonstrated one red (R), two green (G), and two fusion (yellow, Y) signals (1R2G2Y) with the BCL2-IGH DF probe, 1R1G1Y with the BCL2 BA probe, 2R2G2Y with the MYC-IGH DF probe, and 1R1G2Y with the MYC BA probe (Figure 6A).
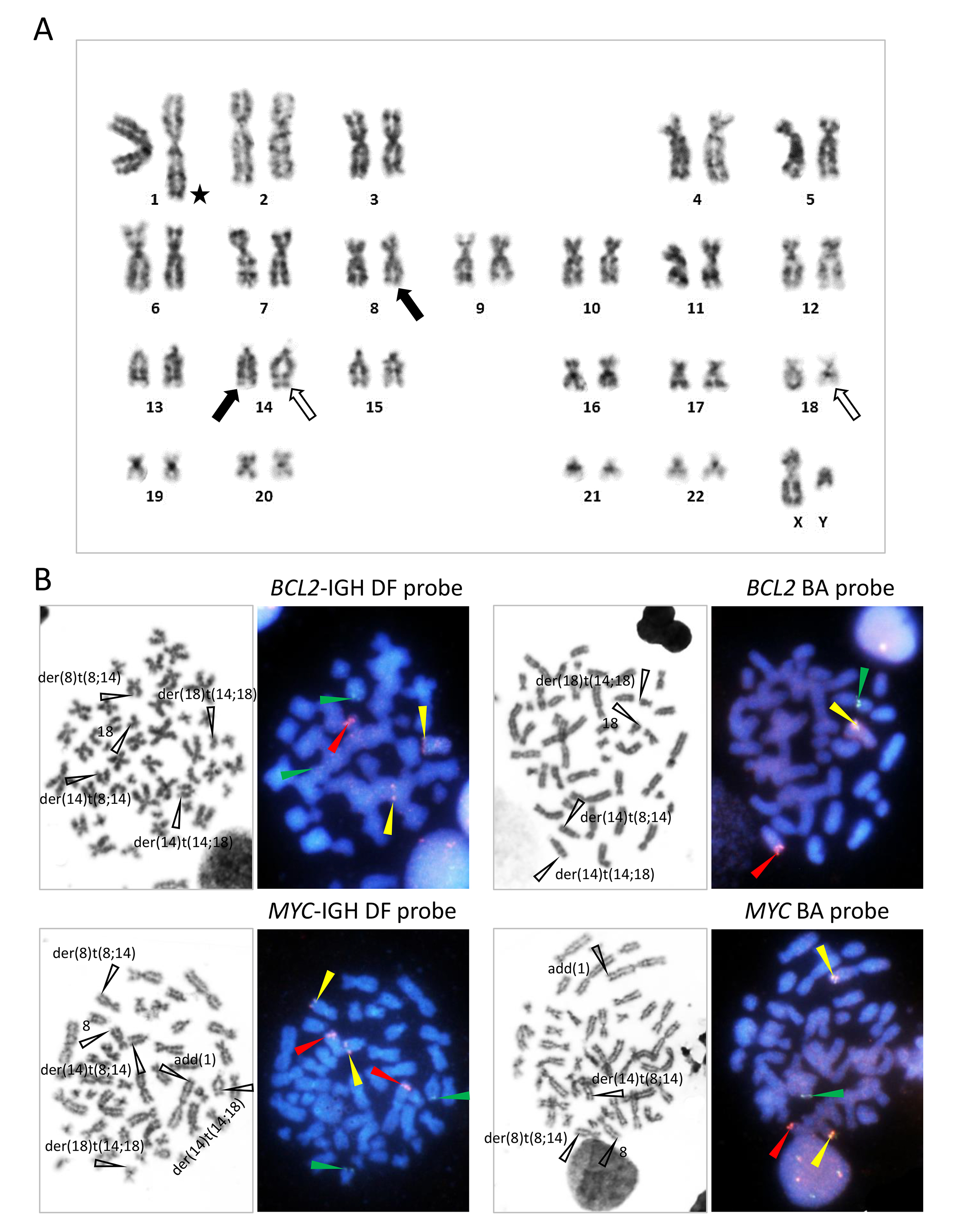
(A) G-banding karyotype obtained from BM. t(8;14)(q24;q32) and t(14;18)(q32;q21) are indicated by closed and open arrows, respectively. An asterisk indicates additional materials of add(1)(q44). The karyotype was 46,XY,add(1)(q44),t(8;14)(q24;q32),t(14;18)(q32;q21). (B) FISH of metaphase spreads using the probes indicated. Pictures of G-banding and FISH through the triple band pass filter are aligned side by side. FISH signals are indicated by arrowheads of respective colors and relevant chromosomes are indicated in the G-banding pictures. FISH probes used are: Vysis LSI IGH/BCL2 Dual Color Dual Fusion Probes (depicted as BCL2-IGH DF probe in the text and figure), Vysis LSI BCL2 Break Apart FISH Probe Kit (BCL2 BA probe), Vysis LSI IGH/MYC/CEP 8 Tri-Color Dual Fusion FISH Probe Kit (MYC-IGH DF probe), and Vysis LSI MYC Dual Color Break Apart Rearrangement Probe (MYC BA probe), all of which were purchased from Abbott Laboratories (Abbott Park, IL, USA).

FISH of interphase nuclei applied to BM (A) and PB (B) specimens. FISH probes are described in the legend of Figure 4. Blue signals may be seen after hybridization with the MYC-IGH DF probe, which contains the SpectrumAqua-labeled 8p11.1-q11.1 CEP8 alpha satellite DNA (asterisks). In B, diploid cell nuclei are shown in the left. The 2R2G2Y signals after the BCL2-IGH DF probe indicative of an extra copy of BCL2 may represent +18, which is often seen in FL with t(14;18)(q32;q21).
To investigate the cytogenetic abnormalities in lymphoma cells in PB, we prepared cytospin smear slides from PB mononuclear cells and hybridized them with the FISH probes. With the BCL2-IGH probe, small-sized nuclei with or without nuclear clefts exhibited the 1R1G2Y signal pattern, indicating the presence of t(14;18)/BCL2-IGH but the absence of t(8;14)/MYC-IGH (Figure 6B, top). Accordingly, these nuclei exhibited the 2R3G0Y pattern with the MYC-IGH DF probe, and occasional nuclei carried 3 R signals indicative of the presence of add(1)(q44) (Figure 6B, bottom). On the other hand, medium-sized nuclei with nuclear clefts showed the 1R2G2Y signal pattern with the BCL2-IGH DF probe and the 2R2G2Y signal pattern with the MYC-IGH DF probe, in accordance with the presence of both t(14;18)/BCL2-IGH and t(8;14)/MYC-IGH in addition to add(1)(q44) (Figure 6B, top and bottom).
We subjected DNA extracted from BM and PB to multiplex polymerase chain reaction (PCR) detecting antigen receptor gene rearrangements and amplifying the junctional sequences of the BCL2-IGH fusion gene.10 As shown in Figure 7, we obtained PCR products of the same size from the two materials, indicative of the rearrangement of IGK and the presence of a fusion gene between the 3′ major break-point region of BCL2 and IGHJ.11
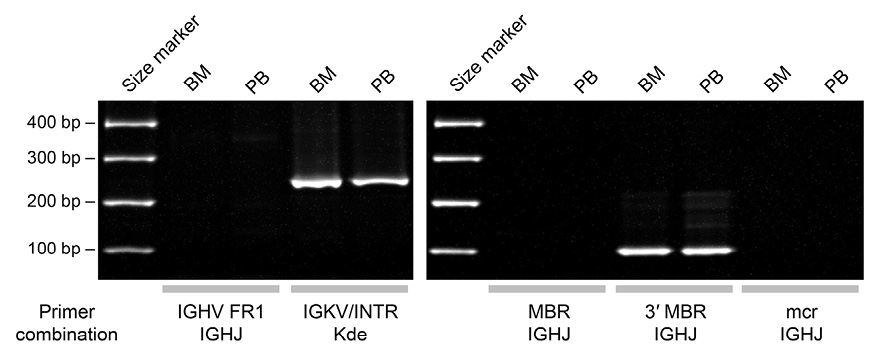
Multiplex PCR showing the clonal rearrangement of IGK (left) and the BCL2-IGH fusion gene (right) in BM and PB lymphoma cells. The PCR products were electrophoresed through polyacrylamide gels and visualized by ethidium bromide staining. Abbreviations: FR1, framework 1; INTR, IGKJ-IGKC intron recombination signal sequence (intronRSS); Kde, kappa deleting element; MBR, major break-point region; mcr, minor cluster region. The sequences of the primers were as described in the BIOMED-2 study.10
We described here a patient with MYC/BCL2 double-hit HGBL who presented with a bulky abdominal tumor and disseminated disease. Lymphoma cells infiltrating into BM exhibited a cytomorphology that mimicked that of BL. In contrast, PB lymphoma cells exhibited indolent cytomorphology compatible to circulating lymphoma cells of FL. Despite the marked difference in cytomorphology, clonal origin of the BM and PB lymphoma cells was confirmed by the fact that these cells shared a common IGK rearrangement and BCL2-IGH fusion sequence.
summarizes cytomorphologic, immunophenotypic, and cytogenetic features of lymphoma cells in BM and PB, excluding small cells in BM due to difficulty in identification using microscopy. BM lymphoma cells were positive for CD10 and expressed CD24 and CD38 at high levels. The presence of t(14;18) and t(8;14) in addition to add(1) in these cells was confirmed by recognizing FISH signals corresponding to each chromosomal abnormality in interphase nuclei. In contrast, the majority of PB lymphoma cells carried t(14;18) but lacked t(8;14) with or without extra copy of MYC, and were negative for CD10 and expression of CD24 and CD38 was absent or at low level; the lack of CD10 expression is consistent with the fact that FL cells are often negative for CD10 in PB.12,13 Therefore, it was indicated that, in this case, indolent FL and MYC/BCL2 double-hit HGBL transformed from FL developed concurrently, even though the sites of the diseases were independent. On the other hand, we found an intermediate cell population in PB in terms of cell size and expression levels of CD10, CD24, and CD38. As the cells carried both t(14;18) and t(8;14), acquisition of t(8;14)/MYC-IGH may not immediately lead to transformation to HGBL, but instead, florid cytomorphologic transformation may occur in BM; BM-restricted BL/lymphoblastic transformation in an FL patient was previously reported.4 Although we were unable to perform histopathological examination of the abdominal tumor, because the tumor exhibited high-level tracer uptake in 18F-FDG-PET/CT study, it is likely that transformed MYC/BCL2 double-hit HGBL cells were proliferating within the tumor.
Site* |
Cell size |
Nucleus |
Cytoplasm |
CD10** |
CD24** |
CD38** |
BCL2-IGH |
Extra copy of MYC |
MYC-IGH |
|---|---|---|---|---|---|---|---|---|---|
BM |
Medium to large |
Round or irregular, dispersed chromatin, one or two nucleoli |
Round or irregular, dispersed chromatin, one or two nucleoli |
Basophilic, vacuoles + |
Bright |
Bright |
+ |
+ |
+ |
PB |
Small to medium |
Cleaved or not cleaved, condensed chromatin, no nucleolus |
Scant |
− |
− |
to dim − |
+ |
− or + |
− |
Medium |
Cleaved, condensed or slightly dispersed chromatin, no nucleolus |
Scant |
Dim |
Dim to + |
+ | + | + | + |
*Cell size was determined by FSC intensities.
**Expression was graded as – (negative), dim, + (positive), or bright compared with reactive B-cells.
FCM evaluation of DHL/THL cells has been described. Some cases lack surface immunoglobulin expression, which may be related to the involvement of multiple IG gene loci by translocations. 1 Decrease in expression of CD20 ranging from dim to absent compared with normal follicle center B cells was seen in 7 of 9 MYC/BCL2 DHL and 1 of 1 MYC/BCL6 DHL. 9 However, a later study showed that CD20 expression levels on DHL/THL cells were variable, ranging from dim to bright in comparison to normal polyclonal B cells, and only a minority of cases was shown to have dim CD20 expression. 14 Nevertheless, concomitant dim expression of CD19 and CD20 was observed exclusively in DHL. 14 In contrast, association of MYC rearrangement and DHL/THL with bright CD38 expression has been consistently observed, 15-18 and this case suggests that CD38 expression is induced by t(8;14)/MYC-IGH and enhanced by cytomorphologic transformation. Finally, we found that the expression level of CD24 also increased in association with transformation from FL to MYC/BCL2 double-hit HGBL. Cross-linking of CD24 molecules induces apoptosis of BL cell 19 ; therefore, it is possible that increased expression of CD24 accounts for the generation of characteristic BL histopathology. We suggest that an FCM panel including CD10, CD24, and CD38 can effectively predict DHL/THL, thereby prompting cytogenetic/FISH studies to determine whether intensive chemotherapy, instead of conventional R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), should be considered.
The authors declare that they have no conflict of interest.
All procedures performed in this study involving the patient were conducted in accordance with the 1964 Helsinki Declaration.
The patient consented to the use of her medical record and clinical materials for research purposes.