2022 Volume 25 Issue 2 Pages 134-138
2022 Volume 25 Issue 2 Pages 134-138

The JAK2 exon 12 mutation. (A) Nucleotide sequences of JAK2 exon 12 (upper case letters, boxed) and flanking regions (lower case letters) (chromosome 9, GRCh38.p14). Amino acids corresponding to exon 12 are indicated by the single letter code under each codon. The positions of nucleotides and amino acids are numbered according to the NCBI Reference Sequence (NM_001322194.2). The sequences of PCR primers are indicated by the horizontal arrows. (B) Electropherogram of direct sequencing of the PCR products, showing the c.1615_1616AA>TT/p.K539L mutation (vertical arrows)
The JAK2 V617F (JAK2 V617F) mutation resulting from a single nucleotide substitution of G to T at position c.1849 (NM_001322194.2) within the JAK2 exon 14 is found in over 95% of patients with polycythemia vera (PV), and thus is a hallmark of this type of myeloproliferative neoplasm (MPN). 1 In the remaining JAK2 V617F-negative PV patients, approximately 3% of cases carry a mutation within a 20-nucleotide region of exon 12. 2,3 In contrast to JAK2 V617F, JAK2 exon 12 mutations include not only single but multiple amino acid substitutions, as well as deletions and duplications with or without amino acid substitutions. 2-4 This report describes a single case of a patient with PV carrying a JAK2 exon 12 mutation who has been treated for over 20 years in our hospital.
A female patient initially presented with erythrocytosis when she was 51 years old. The patient occasionally felt oppression of the anterior chest wall, and high blood pressure had been noted by their family doctor. Blood tests revealed a markedly elevated red cell count, hemoglobin level, and hematocrit value, while the platelet and white cell counts were within the normal ranges (Table 1). White cell differential showed a mild left shift of neutrophils. Abnormal blood chemistry included increased levels of uric acid and lactate dehydrogenase (LDH) (Table 1). The serum erythropoietin (EPO) level was 5.34 mIU/mL (reference range, 7.4–29.8 mIU/mL). The bone marrow showed hypercellularity with 36.5% erythroid series and 51.4% myeloid series (M/E ratio, 1.4), and 250 megakaryocytes were counted per smear slide. No dysplastic morphology was found in the three hematopoietic lineages. The biopsy showed 70 to 80% cellularity. G-banding revealed a normal female karyotype.
|
Variables |
At presentation |
After 20 years |
Reference range |
|---|---|---|---|
Red cells (×106/μL) |
913 |
418 |
386–492 |
Hemoglobin (g/dL) |
24.3 |
9.4 |
11.6–14.8 |
Hematocrit (%) |
76.5 |
29.9 |
35.1–44.4 |
MCV (fL) |
83.8 |
71.5 |
83.6–98.2 |
MCH (pg) |
20.6 |
22.5 |
27.5–33.2 |
MCHC (g/dL) |
31.8 |
31.4 |
31.7–35.3 |
Reticulocytes (%) |
1.7 |
0.3 |
0.6–1.3 |
Nucleated red cells (/100 white cells) |
0 |
1.5 |
0 |
Platelets (×104/μL) |
32.1 |
6.0 |
15.8–34.8 |
White cells (×103/μL) |
8.1 |
19.51 |
3.3–8.6 |
Lymphocytes (%) |
19.0 |
17.0 |
20–45 |
Atypical lymphocytes (%) |
0 |
0.5 |
0 |
Monocytes (%) |
6.0 |
3.5 |
1–7 |
Eosinophils (%) |
0 |
0.5 |
1–3 |
Basophils (%) |
0 |
+ |
0–1 |
Segmented (%) |
68.0 |
40.5 |
45–70 |
Band (%) |
7.0 |
20.0 |
1–3 |
Metamyelocytes (%) |
0 |
7.0 |
0 |
Myelocytes (%) |
0 |
1.0 |
0 |
Promyelocytes (%) |
0 |
1.5 |
0 |
Myeloblasts (%) |
0 |
9.0 |
0 |
Creatinine (mg/dL) |
1.0 |
0.7 |
0.5–0.8 |
Uric acid (mg/dL) |
10.2 |
8.3 |
2.6–5.5 |
Aspartate aminotransferase (U/L) |
66 |
18 |
13–30 |
Alanine transaminase (U/L) |
57 |
13 |
7–23 |
Lactate dehydrogenase (U/L) |
694 |
1,449 |
124–222 |
C-reactive protein (mg/dL) |
0.3 |
12.38 |
0–0.14 |
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
We have developed a sensitive melting-curve assay for detecting the JAK2 V617F mutation and applied it to the differential diagnosis of MPN; 5 however, the DNA extracted from the blood of this patient lacked the mutation. Therefore, we investigated the presence of JAK2 exon 12 mutations by polymerase chain reaction (PCR) and direct sequencing of the PCR products. The PCR primers were designed for intronic sequences encompassing exon 12 (Key Figure A). 3 PCR amplification was performed in a Veriti 96-well thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA), and the PCR products were visualized by ethidium bromide-stained agarose gel electrophoresis. The products were purified using a MinElute® PCR Purification Kit (QIAGEN, Hilden, Germany) and sequenced using a BigDye® Direct Cycle Sequencing Kit and a SeqStudioTM Genetic Analyzer (Thermo Fisher Scientific). As shown in Key Figure B, we found substitutions of two consecutive nucleotides AA to TT at positions c.1615_1616, leading to a single amino acid substitution of lysine to leucine at position p.539 (c.1615_1616AA>TT/p.K539L). The A and T nucleotides represented equivalent electrophoretic peaks, indicating that wild-type and mutant allele burdens were comparable. The JAK2 K539L mutation has reportedly been the 4th most common exon 12 mutation. 2
We first treated the patient with therapeutic phlebotomy to control hematocrit below 55%. The patient was intolerant to interferon due to fatigue and interferon-induced hyperthyroidism. At the age of 58, the patient was diagnosed with type 2 diabetes mellitus, and 12 years after the initial presentation at 63, the patient developed myocardial infarction and received a percutaneous coronary intervention. As the white cell and platelet counts began to increase at that time, hydroxyurea was initiated. The patient received 500 mg of the drug daily to control increasing white cell and platelet counts for 8 years, in addition to antithrombotic, antihypertensive, and antidiabetic medications, and no thrombotic complication or PV-associated symptoms were found.
At the age of 71, 20 years after the initial presentation, the patient presented with headaches to the ER. Blood tests showed that the hemoglobin level had decreased by 5.6 g/dL over 2 months along with an increase in white cell count and LDH level, and the patient was referred to the hematology department. Examination of the blood smear disclosed the presence of nucleated erythrocytes and immature myeloid cells, including blasts, showing the appearance of leukoerythroblastosis (Table 1). Mature neutrophils with pseudo-Pelger nuclear anomaly and those showing hypogranularity were found. The bone marrow aspiration was dry tap. The biopsy revealed marked hypercellularity with MF-3 grade myelofibrosis. Computed tomography showed splenomegaly. DNA extracted from mononuclear cells, including the blasts, in the blood had the c.1615_1616AA>TT/p.K539L mutation (Figure 1). These results indicated that the disease transformed into post-PV myelofibrosis (MF) and evolved into the myelodysplastic/pre-leukemic phase. 1 Thus, we started ruxolitinib at 10 mg twice daily.

The c.1615_1616AA>TT/p.K539L mutation in the blood at the post-PV MF and myelodysplastic/pre-leukemic phase. (A) Total white cells were recovered using an ammonium chloride lysis solution. (B) Mononuclear cells and (C) granulocytes were separated by density gradient stratification (Lymphocyte Separation Solution; Nacalai Tesque, Kyoto, Japan). Genomic DNA was isolated by a column-based procedure (QIAamp DNA Mini Kit; QIAGEN) and purified using magnetic silica beads (MagExtratorTM -Genome-; TOYOBO, Osaka, Japan) for granulocytes, and then subjected to PCR and direct sequencing as described in the text. The AAA to TTA mutated allele burden appears to be higher in the order of granulocytes, total white cells, and mononuclear cells (arrows)
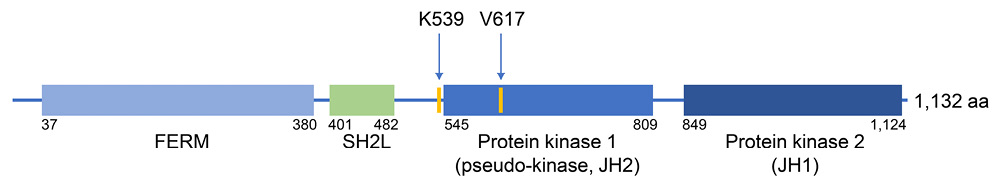
JAK2 encodes a non-receptor tyrosine kinase that serves as the catalytic signaling components for a wide range of cytokine receptors, including the receptor for EPO. 6 JAK2 protein is composed of an N-terminal FERM domain, an SH2-like (SH2L) domain, a kinase-like or pseudokinase domain (JH2), and a C-terminal tyrosine kinase domain (JH1) (Figure 2), and the JH2 functions as a negative regulator of the tyrosine kinase activity of JH1. 6 When the JAK2 V617F mutation occurs within JH2, the inhibitory interaction between JH1 and JH2 domains is disrupted, thereby activating JH1 tyrosine kinase followed by phosphorylation of downstream STAT transcription factors in the absence of EPO binding to the receptor. 6 In contrast, amino acids affected by JAK2 exon 12 mutations are immediately adjacent to JH2 and lie in the linker between SH2L and JH2 domains (Figure 2). 2,4 Since residues affected by exon 12 mutations are mapped close to the V617 residue in a theoretical model of the JAK2 structure, it is proposed that exon 12 mutations also disrupt the JH1-JH2 inhibitory interaction. 2,3 In an experimental model, JAK2 exon 12 mutations resulted in stronger ligand-independent signaling through JAK2, compared with the JAK2 V617F mutation. 3
PV patients with JAK2 exon 12 mutations present at a significantly younger age and exhibit higher hemoglobin levels and hematocrit values, while white cell and platelet counts are significantly lower, compared with JAK2 V617F PV patients. 2 These features were also observed in the current patient (Table 1). Thus, tests for JAK2 exon 12 mutations should be considered for patients who present with isolated erythrocytosis and lack JAK2 V617F. On the other hand, transformation to post-PV MF and evolution to myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) are major causes of mortality in PV patients. 7 In a large international study, approximately 7% of PV patients developed MDS/AML within 20 years. 8 No significant difference was found in the incidence of these hematologic transformations between PV patients with JAK2 V617F and JAK2 exon 12 mutations. 2 Thus, the course of the current patient over 20 years appears to be consistent with the natural progression of PV.