2023 Volume 26 Issue 1 Pages 72-75
2023 Volume 26 Issue 1 Pages 72-75
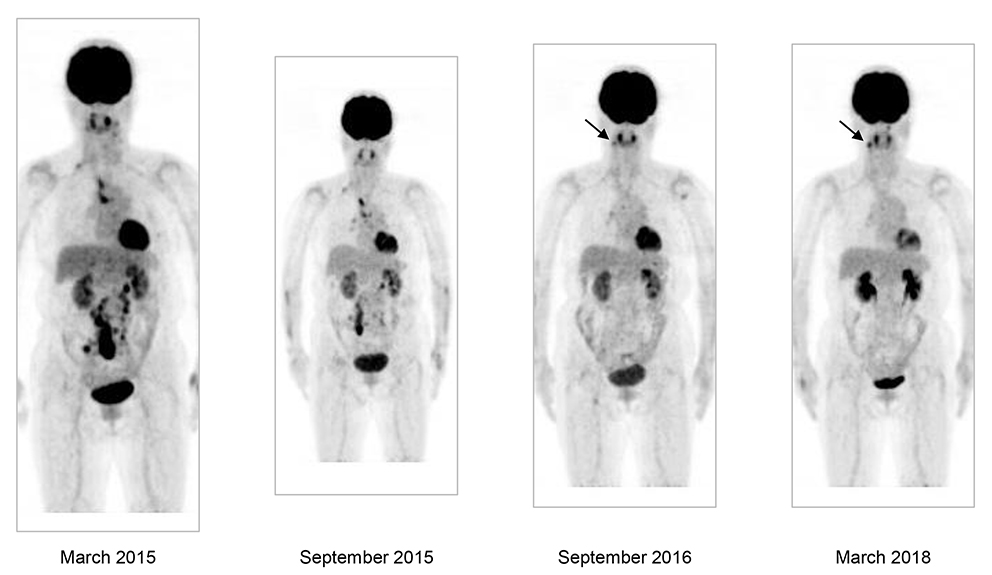
Anterior views of the maximum intensity projection images of 18F-FDG-PET/CT, showing the first scan (left) and those after 6 months (middle, left), after one year and 6 months (middle right), and after 3 years (right). The arrows indicate accumulations reflecting periodontitis of the mandibula.
A woman in her 70s was referred to the Department of Hematology with suspected malignant lymphoma. 18F-fluorodeoxyglucose-positron emission tomography combined with computed tomography (18F-FDG-PET/CT) revealed tracer-avid lymph nodes (LNs) in the left cervical, right supraclavicular, mediastinal paratracheal, abdominal para-aortic, and mesenteric regions, with a maximum standardized uptake value (SUVmax) of 12.5 in the last LN region (Key Figure, left; Figure 1, left). Her hemoglobin level was 13.3 g/dL, white cell count was 4.90 × 103/μL, and platelet count was 197 × 103/μL. The white cell differential was normal. The level of lactate dehydrogenase was 184 U/L (reference range, 100 to 225 U/L), albumin was 4.3 g/dL, uric acid was 5.0 mg/dL, C-reactive protein was <0.2 mg/dL, and soluble interleukin-2 receptor was 406 U/mL (reference range, 145 to 519 U/mL). Her ECOG performance status was 0. She had no constitutional symptoms.

Axial views of representative PET/CT fused images of 18F-FDG-PET/CT, showing the first scan (left) and those after 6 months (middle, left), after one year and 6 months (middle right), and after 3 years (right).
She underwent biopsy of the para-aortic LNs under retroperitoneal laparoscopy in the Department of Urology.1 Pathological specimens prepared from the biopsy were composed of neoplastic follicles that varied in size and shape, and the follicular growth pattern was highlighted by anti-CD21 immunostaining for follicular dendritic cells (Figure 2A and B). Higher magnification examination revealed a mixture of medium-sized and large cells, corresponding to the grade 1–2 category of follicular lymphoma (FL) (Figure 2C). Lymphoma cells were positive for CD10, CD20, CD79a, and BCL2 and negative for CD3 (Figure 2D, E, and F) by immunohistochemistry and CD19+, CD20+, CD21dim, CD22dim, CD23−, CD24+, CD5−, CD10+, CD38+, CD45RO+, HLA-DR−/dim, and IgG/λ+ by flow cytometry. G-banding revealed t(14;18)(q32;q21) with duplication of der(18)t(14;18) and loss of the normal homologue of chromosome 18 (Figure 3A). Accordingly, interphase nuclei hybridized with the BCL2/IGH dual color, dual fusion probe (Abbott Laboratories, Abbott Park, IL, USA) were labeled by zero red (R, BCL2), one green (G, IGH), and three yellow (Y, BCL2::IGH fusion) signals (0R1G3Y) (Figure 3B).

Histopathology of the LN biopsy. A, Hematoxylin and eosin (H&E) staining (original magnification of the objective lens, 10×); B, anti-CD21 immunostaining (10×); C, H&E (40×); D, anti-CD20 (20×); E, anti-CD10 (20×); and F, anti-BCL2 (20×).
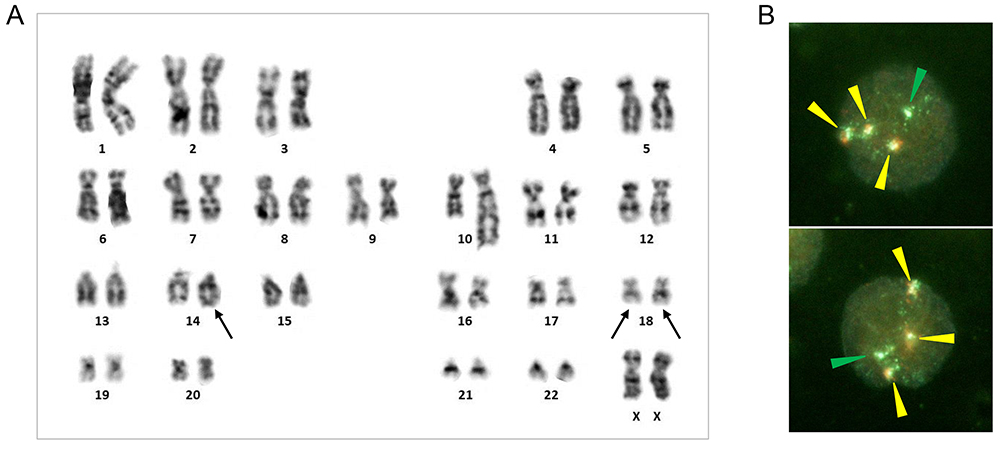
Cytogenetic studies. (A) G-banding karyotype. t(14;18)(q32;q21) and +der(18)t(14;18)(q32;q21) are indicated by arrows. The karyotype is 46,XX,add(10)(q22),t(14;18)(q32;q21),−18,+der(18)t(14;18)(q32;q21). (B) Interphase FISH using the BCL2/IGH dual color, dual fusion probe, showing the 0R1G3Y hybridization signal pattern (arrowheads of each color).
Because the disease matched the criteria for the low-tumor burden category of FL,2 we adopted a watchful waiting policy. A second 18F-FDG-PET/CT performed 6 months after the first scan demonstrated a distribution of involved LNs similar to that of the first scan, but the abdominal lesions became smaller and their FDG accumulation level was reduced, with a SUVmax of 9.34 (Key Figure, middle, left; Figure 1, middle, left). A third scan 12 months after the second one showed further reduction of the avidity in the mediastinal, abdominal para-aortic, and mesenteric LNs, leaving only weak tracer accumulations in the right supraclavicular, paratracheal, and right hilar LNs (Key Figure, middle, right; Figure 1, middle, right). In a fourth scan, performed one and a half years after the third one, accumulations of the tracer in the right supraclavicular, paratracheal, and right hilar LNs were reduced to an undetectable level (Key Figure, right; Figure 1, right). Currently, no obvious lymphoma lesions can be observed, 8 years after the LN biopsy.
Here, we described a female patient with stage III FL carrying t(14;18)(q32;q21)/IGH::BCL2, which is the hallmark of this type of low-grade B-cell lymphoma.3 Her disease spontaneously regressed after biopsy without lymphoma-directed therapy, the course of which was well-illustrated by serial 18F-FDG-PET/CT, and she has remained lymphoma-free for a prolonged period. Patients with advanced-stage FL do not necessarily require immediate treatment. The adenopathy commonly waxes and wanes spontaneously and some patients demonstrate stable disease for years. Rarely, patients may show spontaneous remission lasting longer than one year4-6; in an earlier report, 18 (12.8%) of 140 FL patients showed spontaneous regression.7 We clinicians sometimes observe a reduction in size of adjacent LNs after LN biopsy, and consider that an immune response against the tumor has been elicited, although transiently, after a surgical procedure. The current patient was complicated by large retroperitoneal fluid collection due to lymphorrhea after biopsy and underwent drainage treatment as well as additional retroperitoneal laparoscopic surgery to seal the lymphatic leakage with fibrin glue. Thus, it is possible that a long-term immune response against the tumor may have been induced after these surgical procedures, thereby leading to complete regression of the tumor.
The author is grateful to Dr. H. Kawanishi of the Department of Urology for conducting the LN biopsy under retroperitoneal laparoscopy, Dr. T. Misaki of the Radioisotope Center for 18F-FDG-PET/CT, and Dr. S. Sumiyoshi of the Department of Diagnostic Pathology for pathological diagnosis.