2013 年 41 巻 3 号 p. 95-102
2013 年 41 巻 3 号 p. 95-102
Infections by virulent strains of Vibrio parahaemolyticus are frequently reported in Southeast Asia. This is due to the frequent seafood contamination by virulent strains. In this study conducted from 2008 to 2011, seafood like fish, shrimp, squid, crab, and molluscan shellfish were purchased from provinces in Thailand and three Southeast Asian countries and examined for the prevalence of three genetic markers of V. parahaemolyticus (species-specific gene: toxR gene, virulence genes: tdh and trh genes). An enrichment culture of seafood was examined for these markers using PCR methods. Molluscan shellfish showed a high frequency of contamination in Thailand. The shellfish harvested from the Gulf of Thailand were significantly more contaminated with virulence genes than those from the Andaman Sea. The seafood purchased from three Southeast Asian countries was positive for the three markers of V. parahaemolytcus at differing frequencies. The virulence markers (tdh and trh markers) were frequently detected in molluscan shellfish from Vietnam (17.9 and 8.0%, respectively), Malaysia (11.1 and 16.7%), and Indonesia (9.1 and 13.6%). These data suggest that the molluscan shellfish sold in Southeast Asian markets are highly contaminated with virulent strains of V. parahaemolyticus.
Diarrhea is a serious public health issue in developing countries. The prevention requires strict hygiene related to drinking water and food in these countries. Vibrio parahaemolyticus infection is caused by consumption of raw or partially cooked seafood contaminated with virulent strains of V. parahaemolyticus. V. parahaemolyticus is a gram-negative and halophilic bacterium. This bacterium inhabits marine and estuarine environments and appears at water temperatures above 15°C. Additionally, this bacterium is one of the most important food-borne pathogens in tropical and subtropical areas. The virulence factors of V. parahaemolyticus are a thermostable direct hemolysin (TDH) and a TDH-related hemolysin (TRH) coded by the tdh gene and the trh gene, respectively [1]. TDH is responsible for the Kanagawa phenomenon, that is, beta-hemolysis on Wagatsuma agar. Almost all V. parahaemolyticus isolates from clinical specimens possess the virulence markers (tdh and trh genes), while environmental strains isolated from seawater and seafood seldom possess these genes [2, 3]. The proportion of virulent strains among environmental isolates detected using the Kanagawa phenomenon was approximately 1% [4].
The emergence of pandemic strains of V. parahaemolyticus has been reported from diarrhea patients in Asian countries since 1996 [5, 6]. The clonality of the pandemic strains was confirmed using an arbitrary-primed PCR. A simple PCR method for detecting the pandemic strains utilizes unique nucleotide changes in the toxRS operon of the pandemic strains [6]. This method was useful for surveys and the epidemiological analysis of pandemic stains. This method was also used to confirm the pandemic spread to North America, South America, Europe, and Africa [6–9]. Furthermore the seafood contamination by the pandemic strain was reported in Thailand [10]. During this study on the pandemic strains, a high frequency of infection by virulent strains of V. parahaemolyticus was reported [11, 12].
Southeast Asian countries export seafood to developed countries, and its transportation vary and are seldom reported. Raw food contaminated with food-borne pathogens and antibiotic-resistant bacteria have been studied in Southeast Asia [13–15]. Recently, seafood becomes popular worldwide because it is considered to be healthy, and this has resulted in an increase in both production and domestic consumption of seafood in Southeast Asia [16]. It is important to determine the frequency of seafood contamination by the virulent strains of V. parahaemolyticus in the region, but no such comparative studies across countries have been reported. As Vuddhakul et al. reported, only molluscan shellfish harbored virulent strains in southern Thailand as compared to various other types of seafood [17].
In this study, I investigated and compared the incidence of V. parahaemolyticus in various areas of Thailand and three Southeast Asian countries. The incidence of V. parahaemolyticus in seafood may be influenced by natural causes as well as by socio-economic trends and other factors in the human environment.
Annual fishery yields were compiled using statistics data from the Department of Fisheries of Ministry of Agriculture and Cooperatives of Thailand.
Study area and sample collectionThailand consists of 76 provinces and Bangkok Metropolis. There are officially five coastal zones containing 23 provinces and Bangkok Metropolis (Fig. 1). Seafood samples were purchased from provinces in each coastal zone between 2008 and 2011 for the study in Thailand (Table 1). For a comparative study in Southeast Asia, seafood samples were also purchased in Hanoi, Vietnam; Selangor, Malaysia; Padang and Jakarta, Indonesia between 2009 and 2011. The collected samples were transferred to sterile plastic bags.
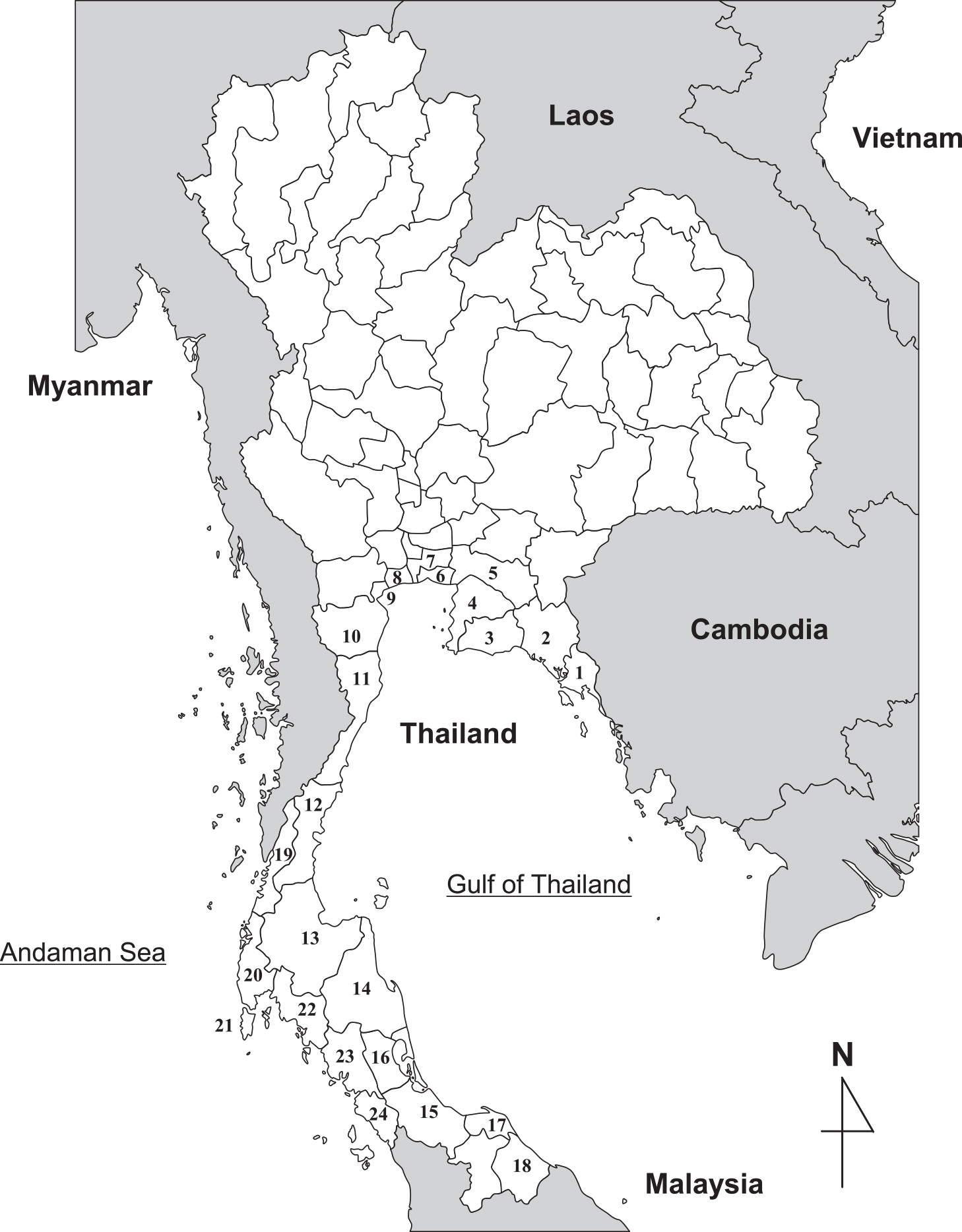
Provinces corresponding to coastal zone 1 to 5 designated by the Thai government.
Coastal zone 1: Trat (1), Chanthaburi (2), and Rayong (3). Coastal zone 2: Chon Buri (4), Chachoengsao (5), Samut Prakan (6), Bangkok (7), Samut Sakhon (8), Samut Songkhram (9), and Phetchaburi (10). Coastal zone 3: Prachuap Khiri Khan (11), Chumphon (12), and Surat Thani (13). Coastal zone 4: Nakhon Si Thammarat (14), Songkhla (15), Phatthalung (16), Pattani (17), and Narathiwat (18). Coastal zone 5: Ranong (19), Phangnga (20), Phuket (21), Krabi (22), Trang (23), and Satun (24).
| Coastal zone and Province (No in Figure 1) | No. of examined molluscan shellfish*: | Oriental hard clam | ||
|---|---|---|---|---|
| Bloody cockle | Green mussel | Oyster | ||
| Coastal zone 1 | 14 | 11 | 5 | 8 |
| Trat (1) | 4 | 5 | ||
| Chanthaburi (2) | 14 | 7 | ||
| Rayong (3) | 8 | |||
| Coastal zone 2 | 49 | 47 | 11 | 36 |
| Chon Buri (4) | 8 | 6 | 11 | 11 |
| Samut Prakan (6) | 16 | 20 | 8 | |
| Samut Sakhon (8) | 10 | 7 | 13 | |
| Samut Songkhram (9) | 9 | 12 | 4 | |
| Petchaburi (10) | 6 | 2 | ||
| Coastal zone 3 | 13 | 16 | 13 | |
| Surat Thani (13) | 13 | 16 | 13 | |
| Coastal zone 4 | 11 | 9 | 14 | |
| Songkhla (15) | 8 | |||
| Pattani (17) | 11 | 9 | 6 | |
| Coastal zone 5 | 22 | 6 | 15 | |
| Krabi (22) | 4 | |||
| Trang (23) | 4 | 6 | ||
| Satun (24) | 18 | 11 | ||
| Total | 109 | 73 | 32 | 86 |
* Bloody cockle (Anadara granosa), green mussel (Perna viridis), oyster (Crassostrea belcheri and Saccostrea commercialis), and Oriental hard clam (Meretrix mereitrix, M. casta, M. casta, and M. casta)
A 10-g portion of seafood sample was mixed with 90 ml alkaline peptone water (APW; Nissui Co., Tokyo, Japan) in a stomacher bag and gently homogenized by manual shaking. The homogenate was transferred into a sterile tube and incubated at 37°C for 18 hrs. A 1-ml aliquot of APW enrichment culture was centrifuged at 10,000 × g for 3 min. After the supernatant was removed, the pellet was re-suspended in 1 ml sterilized saline water and heated at 100°C for 5 min. After centrifugation at 12,000 × g for 5 min, the boiled supernatant was collected for the PCR assay. The PCR assays for detection of V. parahaemolyticus species-specific toxR gene and virulence genes (tdh and trh) were performed as described previously [18, 19].
Molluscan shellfish is a popular seafood in the coastal areas of Thailand, and three kinds of molluscan shellfish, i.e. bloody cockle (Anadara granosa), green mussel (Perna viridis), and oysters (Crassostrea belcheri and Saccostrea commercialis), are cultured in these coastal areas (Fig. 1). The harvest yield began to show an increase in 1998 for bloody cockle, 1999 for green mussel, and 1993 for oysters (Fig. 2). As for the harvest of molluscan shellfish in 2008, the major harvest area was in coastal zone 2 (41%) and 3 (53%) for bloody cockle, coastal zone 2 (89%) for green mussel, and coastal zone 1 (38%) and 3 (54%) for oysters, showing that coastal zones 2 and 3 are the major harvest areas of molluscan shellfish in Thailand (Fig. 3).

Annual production of each molluscan shellfish species in Thailand from 1988 to 2008: bloody cockle (Anadara granosa) (A), green mussel (Perna viridis) (B), and oysters (Crassostrea belcheri and Saccostrea commercialis) (C). The statistical data were collected and compiled from the web site (http://www.fisheries.go.th/it-stat/) and compiled.

Production of molluscan shellfish in coastal zones in 2008: bloody cockle (A), green mussel (B), and oysters (C). The statistical data were collected from the web site (http://www.fisheries.go.th/it-stat/). See Figure 1 legend for designation of coastal zones.
The coastal areas of Thailand can be divided into two regions, one facing the Gulf of Thailand (abbreviated as Region GT in this paper, corresponding to coastal zones 1 to 4 in Fig. 1) and the other facing the Andaman Sea (abbreviated as Region AS in this paper, corresponding to coastal zone 5 in Fig. 1). The environmental conditions differ in these two regions. Seafood samples were purchased from both GT and AS, and the incidence of V. parahaemolyticus virulence markers in fish, squid, shrimp, crab, and molluscan shellfish was analyzed by using PCR methods. As for the virulence markers, 40 (tdh gene) for 11 (trh gene) samples were positive in 300 molluscan shellfish (Table 2). But none of the 82 seafood samples including 32 fish, 13 squid, 26 shrimp, and 11 crabs was positive (data not shown). The species of molluscan shellfish collected in the various coastal zones are summarized in Table 2. The detection of the three markers for V. parahaemolyticus is classified for each kind of molluscan shellfish (Table 2). The overall incidence of the marker (toxR gene) for V. parahaemolyticus in bloody cockle, green mussel, oysters, and oriental hard clams (Meretrix mereitrix, M. casta, M. casta, and M. casta) was 63.3, 69.9, 12.5, and 74.4%, respectively (Table 2). The overall incidence of the tdh gene and trh gene in the four kinds of molluscan shellfish was 6.4, 16.4, 0, 24.4% and 2.8, 4.1, 0, 5.8%, respectively. The data shows that oriental hard clams and green mussels are contaminated at high levels with the tdh gene-bearing V. parahaemolyticus. The level of contamination for the trh gene-bearing V. parahaemolyticus was lower than that for the tdh gene in the molluscan shellfish. Compared with other molluscan shellfish, the contamination by V. parahaemolyticus for oysters was very low (Table 2).
| Coastal zone | Region* | Total | Bloody cockleIncidence of marker gene: | Total | Green musselIncidence of marker gene: | Total | OysterIncidence of marker gene: | Total | Oriental hard clamIncidence of maker gene: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| toxR | tdh | trh | toxR | tdh | trh | toxR | tdh | trh | toxR | tdh | trh | ||||||
| 1 | GT | 14 | 8 (57.1)** | 0 | 0 | 11 | 8 (72.7) | 1 (9.1) | 0 | 5 | 0 | 0 | 0 | 8 | 6 (75.0) | 2 (25.0) | 1 (12.5) |
| 2 | GT | 49 | 34 (69.4) | 4 (8.2) | 2 (4.1) | 47 | 35 (74.5) | 9 (19.1) | 2 (4.3) | 11 | 2 (18.2) | 0 | 0 | 36 | 30 (83.3) | 13 (36.1) | 2 (5.6) |
| 3 | GT | 13 | 9 (69.2) | 1 (7.7) | 0 | NT*** | NT | NT | NT | 16 | 2 (18.2) | 0 | 0 | 13 | 9 (69.2) | 3 (23.1) | 1 (7.7) |
| 4 | GT | 11 | 7 (63.6) | 2 (18.1) | 1 (9.1) | 9 | 6 (66.7) | 2 (22.2) | 1 (11.1) | NT | NT | NT | NT | 14 | 10 (71.4) | 3 (21.4) | 0 |
| 5 | AS | 22 | 11 (50.0) | 0 | 0 | 6 | 2 (33.3) | 0 | 0 | NT | NT | NT | NT | 15 | 9 (60.0) | 0 | 1 (6.7) |
| Total | 109 | 69 (63.3) | 7 (6.4) | 3 (2.8) | 73 | 51 (69.9) | 12 (16.4) | 3 (4.1) | 32 | 4 (12.5) | 0 | 0 | 86 | 64 (74.4) | 21 (24.4) | 5 (5.8) | |
* Region GT faces the Gulf of Thailand and Region AS is faces the Andaman Sea (Figure 1).
** Positive % of total samples in parentheses.
*** Not tested
The overall incidence of V. parahaemolyticus in coastal zones 1 to 4 (57.1 to 69.4% for bloody cockle; 66.7 to 74.5% for green mussel; 69.2 to 83.3% for oriental hard clams) was higher than that in coastal zone 5 (50.0, 33.3, and 60.0%). The overall incidence of the tdh gene in coastal zones 1 to 4 (0 to 36.1%) was the same or higher than that in coastal zone 5 (0%). Likewise, the overall incidence of the trh gene in coastal zones 1 to 4 (0 to 12.5%) was the same or higher than that for costal zone 5 (0 to 6.7%). The data suggests that the incidence of three markers is lower in Region AS (coastal zone 5) than in Region GT (coastal zones 1 to 4).
Prevalence of contamination by V. parahaemolyticus and virulence genes present in seafood marketed in Southeast Asian countriesA total of 74, 52, and 130 seafood samples were purchased from wet markets and supermarkets in Vietnam, Malaysia, and Indonesia (Table 3). In Vietnam, the level of contamination by V. parahaemolyticus-specific markers for fish, shrimp, squid, crab, and molluscan shellfish was 62.5, 72.2, 28.6, 40.0, and 89.2%, respectively (Table 3). The incidence of tdh and trh markers in molluscan shellfish was 17.9 and 8.0%, respectively (Table 3). In Malaysia, the level of contamination by V. parahaemolyticus in five kinds of seafood was 54.5, 54.5, 22.2, 0, and 66.7%, respectively (Table 3). The incidence of tdh and trh markers in molluscan shellfish was 11.1 and 16.7% (Table 3). In Indonesia, I investigated in two places. The level of contamination by V. parahaemolyticus in fish, shrimp, and squid was 56.5, 66.7 and 15.4%, respectively, in Padang. The level of contamination by V. parahaemolyticus in fish, shrimp, squid, crab, and molluscan shellfish was 53.3, 57.9, 12.5, 0, and 72.7%, respectively, in Jakarta (Table 3). The incidence of tdh and trh markers in molluscan shellfish was 9.1 and 13.6%, respectively, in Jakarta (Table 3). The incidence of the trh marker is the same or higher than that of the tdh marker in Indonesian seafood. The virulence marker-positive colonies were isolated and the virulence genes for them were confirmed (data not shown).
| Country (Location) | Group of Seafood | No. of samples examined | Incidence of marker gene: | ||
|---|---|---|---|---|---|
| toxR | tdh | trh | |||
| Vietnam (Hanoi) | Fish | 16 | 10 (62.5)* | 0 | 0 |
| Shrimp | 18 | 13 (72.2) | 0 | 0 | |
| Squid | 7 | 2 (28.6) | 0 | 0 | |
| Crab | 5 | 2 (40.0) | 0 | 0 | |
| Shellfish | 28 | 25 (89.2) | 5 (17.9) | 2 (8.0) | |
| Total | 74 | 52 (70.3) | 5 (6.8) | 2 (2.7) | |
| Malaysia (Selangor) | Fish | 11 | 6 (54.5) | 0 | 0 |
| Shrimp | 11 | 6 (54.5) | 0 | 0 | |
| Squid | 9 | 2 (22.2) | 0 | 0 | |
| Crab | 3 | 0 | 0 | 0 | |
| Shellfish | 18 | 12 (66.7) | 2 (11.1) | 3 (16.7) | |
| Total | 52 | 26 (50.0) | 2 (3.8) | 3 (5.8) | |
| Indonesia (Padang) | Fish | 23 | 13 (56.5) | 0 | 2 (8.7) |
| Shrimp | 18 | 12 (66.7) | 0 | 3 (16.7) | |
| Squid | 13 | 2 (15.4) | 0 | 0 | |
| Total | 54 | 27 (50.0) | 0 | 5 (9.3) | |
| Indonesia (Jakarta) | Fish | 15 | 8 (53.3) | 0 | 0 |
| Shrimp | 19 | 11 (57.9) | 1 (5.3) | 1 (5.3) | |
| Squid | 16 | 2 (12.5) | 0 | 0 | |
| Crab | 4 | 0 | 0 | 0 | |
| Shellfish | 22 | 16 (72.7) | 2 (9.1) | 3 (13.6) | |
| Total | 76 | 37 (48.7) | 3 (3.9) | 4 (5.3) | |
* Rate (%) of positive sample per total samples in parentheses
Seafood is gaining popularity worldwide because it is considered to be healthy. However, since the supply of seafood from natural resources is limited, culture-based sources make up for its shortage in many countries. Large quantities of seafood are produced by the culture method not only for domestic consumption but also for export. To ensure its safety and quality, it is necessary to determine whether the seafood produced and sold is contaminated with virulent strains of V. parahaemolyticus or not.
I confirmed that molluscan shellfish of various types are highly contaminated with virulent strains of V. parahaemolyticus [10, 17]. The production of cultured shellfish increased during the 1990s in Thailand (Fig. 2). According to the recent trends in the production of these shellfish, it is indicated that Thai people prefer three kinds of molluscan shellfish (bloody cockle, green mussel, and oysters). The molluscan shellfish is eaten after cooking by various methods. The two shellfish, green mussel and oriental hard clam, are well cooked, while only bloody cockle is undercooked. There are two kinds of oyster in Thailand. One is a small oyster (Saccostrea commercialis) that is harvested from coastal zones 1 and 2 (Fig. 1) and well cooked before eating. The other is a large oyster (Crassostrea belcheri) that is harvested from coastal zone 3 (mainly Surat Thani designated as No. 13 in Fig. 1) and eaten raw. The harvest areas (coastal zones 2 and 3) where the yield of molluscan shellfish has increased are near Bangkok Metropolis (Fig. 3). The high level of virulence markers shows that the molluscan shellfish harvested in these areas is highly contaminated with virulent strains of V. parahaemolyticus and pose a high risk of V. parahaemolyticus infection.
The data shown in Table 2 demonstrates the difference in contamination of molluscan shellfish for markers of V. parahaemolyticus and the virulence genes in Regions GT and AS. The data supports the idea that the environment of Region GT (coastal zones 1 to 4) may be more suitable for the survival and/or growth of V. parahaemolyticus. This may be influenced by the natural environment (geographical features, climate change, etc.) and human environment (eutrophication, etc.) in these regions.
Here I compared the incidence of V. parahaemolyticus and virulent strains in three countries of Southeast Asia. This is the first report to show the high frequency of contaminated seafood in Southeast Asia. The seafood contamination by V. parahaemolyticus, as indicated by the toxR marker, is higher in Vietnam than in Malaysia or Indonesia (Table 3). With regard to the incidence of virulence markers in molluscan shellfish, the tdh marker was higher in Vietnam than in Malaysia or Indonesia (Table 3). However, the incidence of trh marker in Vietman was lower than that in the two other countries, suggesting that molluscan shellfish in Vietnam are contaminated with tdh-bearing V. parahaemolyticus at high levels but with trh-bearing V. parahaemolyticus at low levels. The contamination of molluscan shellfish by tdh-bearing V. parahaemolyticus is also high in Malaysia. The contamination of molluscan shellfish by the trh-bearing V. parahaemolyticus is high in both Malaysia and Indonesia. These findings indicate that tdh-bearing V. parahaemolyticus inhabits mainland Southeast Asia (Thailand, Vietnam, and Malaysia) while trh-bearing V. parahaemolyticus inhabits maritime Southeast Asia (Indonesia). However, the data are not enough because of a limited sample, location, and time. Therefore, a further studies on a larger scale are needed to confirm the findings.
Not only in Thailand but also in other Southeast Asian countries, virulent strains of V. parahaemolyticus are likely to be confirmed at a high frequency in molluscan shellfish. This may be due to the increased urbanization in Thailand and other Southeast Asian countries. The seafood contamination through urbanization may expand to other parts of the world through the active export trade from Southeast Asian countries.
I am grateful to Prof. Mitsuaki Nishibuchi in Kyoto University for insightful comments, Prof. Son Radu in Putra Malaysia University for tremendous support, Prof. Varaporn Vuddhakul in Prince of Songkla University for enormous help, Dr. Nguyen Binh Minh in National Institute of Hygiene and Epidemiology for invaluable help, and Dr. Abdul Aziz Djamal and Prof. Marlina in Andalas University for long-term support.
This study was supported in part by the Connecting the Region to the World in Southeast Asian Studies: Internationalizing young scholars of The Institutional Prpgram for Younger Researcher Overseas Visitis and a Grant-in-Aid for Scientific Research (KAKENHI 19101010) from the Japan Society for the Promotion of Sciences.