Abstract
Leptospirosis is a worldwide zoonosis and common in tropical and subtropical areas with high rainfall. It should be noted as an imported infectious disease although it is sporadic in Japan. Some imported cases already have been reported in Japan and these cases occurred mainly in Southeast Asia. The case discussed in this article is the first reported Japanese case infected in Vietnam. Four days after returning back to Japan after a two-week stay in the mountain area near Hue, in the middle part of Vietnam, the patient suddenly experienced chills, a high fever, sore throat, gastrocnemius pain, and headache. Conjunctival jaundice, renal function disorder, and proteinuria were observed on the third day of onset. Significant increase in antibody titers against serovar Australis and Autumnalis strains was observed in paired serum samples by microscopic agglutination test (MAT). Consequently we recognized this case as a diagnosis of severe leptospirosis (Weil’s disease). Finally, renal function disorder did not deteriorate further, and then the patient recovered after the tenth day of onset with the administration of antibiotics and supportive care without sequelae. We experienced the first imported Japanese case of severe human leptospirosis infection from Vietnam that was successfully treated with ceftriaxone and minocycline.
Introduction
Leptospirosis is a worldwide zoonosis caused by infection with pathogenic spirochetes of the genus Leptospira. Rodents (particularly rats) are the main hosts of leptospires that may infect humans. However, many mammals can be maintenance hosts of leptospires. After the host is infected, the leptospires replicate in the host’s renal tubules and are excreted in the urine over a long period. Pathogenic leptospires can survive in urine-contaminated water and soil for several weeks, which is an extremely important factor with regard to transmission. People are exposed to a significant risk of leptospire infection by swimming or bathing in water areas in the affected regions, working closely with infected animals, or working in an environment heavily contaminated with infected urine. Outdoor activities, including athletic events, are also important risk factors. In the past decade, leptospirosis has emerged as a globally important infectious disease. It occurs in urban environments of industrialized and developing countries, as well as in rural regions worldwide. It is especially endemic in tropical and sub-tropical regions, and it should be noted that large epidemics often occur after disastrous rainfall and floods in those areas [1]. Looking at the current situation of leptospirosis in Japan, a total of 78 domestic cases were reported from 1999 to 2003 [2]. Fifty-three of the cases (67.9%) were reported in Okinawa Prefecture. After the launch of a national surveillance program for leptospirosis, 137 cases, including imported cases, were reported from 2003 to 2008 [3]. Imported cases reported in Japan were caused by infections from Thailand, Malaysia (Borneo Island), and Indonesia (Bali Island). No imported cases of infection from Vietnam have been reported to date. The infection trigger of these imported cases was swimming or bathing in rivers in the aforementioned countries. Clinical presentation of leptospirosis includes a variety of symptoms from mild to severe, and mortality remains significant in severe cases, a situation related to delays in diagnosis due to lack of infrastructure and adequate clinical suspicion. Pulmonary hemorrhage is increasingly recognized as a major, often fatal, manifestation of leptospirosis—the pathogenesis of which remains unclear. The main treatment is still the administration of tetracycline and β-lactam/cephalosporin. Preventive measures are vaccine and chemoprophylaxis. A vaccine against four serotypes (Copenhageni, Australis, Autumnalis, Hebdomadis) is available in Japan, but this vaccine is not widely recognized as a preventative measure. Prevention is largely dependent on sanitation measures that may be difficult to implement in many countries and regions where infection is endemic. We encountered the first imported Japanese case of leptospirosis infection from Vietnam. In this article, we report the clinical progress of the case and conduct an analysis of the imported cases of leptospirosis in Japan.
Case Presentation
The case under study is that of a 59 year-old Japanese male staff member of an academic institution. He camped in the mountains near Hue, located in the north-central part of Vietnam, for five days in the middle of May, drinking the river water and bathing in the river there. Before bathing in the river, he was also attacked by leeches. Three days after returning to Japan, he started to feel a shaking chill and became febrile. His body temperature rose to 38.8°C, and he developed a headache and sore throat. He visited a clinic where cephem antibiotic and NSAID were prescribed on the basis of a diagnosis of an upper respiratory infection on June 1st. However, his condition did not improve. Therefore, on the next day he visited the outpatient department of our hospital with general fatigue, headache, sore throat and sore back muscle. The physical examination revealed conjunctival jaundice and gastrocnemius tenderness. The patient was then admitted to our hospital for assessment and treatment.
Regarding travel history, the patient had been to China (Yunnan Province), Taiwan, Madagascar, Malaysia (Borneo island), and Thailand. He had never suffered from malaria before. The medical history showed an elevation of serum γ-GTP (around 120 IU/L) and mildly high blood pressure, as well as a history of allergy to mealworm and some kinds of metal substances, but no specific allergy to drugs. The patient did not smoke cigarettes but drank several times a week. He had not received any inoculations before travel. The patient had been bitten by mosquitoes at the camp site in Vietnam, but he did not take any chemoprophylaxis against malaria infection.
Upon physical examination, the patient’s consciousness was clear and his blood pressure was 138/93 mmHg, with a pulse of 86 beats per minute, and respiration was 18 breaths per minute. The oxygen saturation was 97% in the ambient air. His body temperature was elevated to 39.1°C, his height was 172.4 cm, and weight was 80.6 kg (BMI = 27.1). There were some scars on his lower body where leeches had been attached (Figure 1). The sclerae were icteric and the pharynx was reddened. Photophobia was not observed. Chest examination revealed normal cardiac sound and normal lung sound. There was no lymphadenopathy or hepatosplenomegaly. Bowel sound was weakened. Neurologic examination revealed no abnormal findings. Grip pain on gastrocnemius muscle was observed bilaterally, and small petechia was observed on the left lower leg. On the blood examination, the white blood cell count was elevated and the neutrophil count occupied more than 96%, while thrombocytopenia was not observed. The value of total bilirubin was elevated to 2.2 mg/dL (Table 1). The laboratory tests for hepatitis A, hepatitis B, hepatitis C, syphilis and HIV infection were all negative. The results of rapid diagnostic tests were negative for malaria and dengue. The analysis of the Giemsa-stained peripheral-blood thin smear (pH = 7.2) showed no malaria-infected erythrocytes. We repeated the examination several times when he had fever. Simultaneously, a stool examination (thick film, concentrative method) was conducted, but neither eggs nor cysts were detected. No spirochetes could be detected in either the blood or the urine smear. Bacterial colonies did not grow in either the stool culture or the blood culture. The equipment of Korthof medium and EMJH medium were not available in the aforementioned hospital. Urine output decreased to less than 800 mL/day, and proteinuria was detected. Serum urea nitrogen and creatinine levels were elevated to 33 mg/dL and 3.04 mg/dL respectively. These results worsened after hospital admission, indicating that the patient suffered acute renal function disorder. Other potential illnesses such as malaria and dengue were excluded. Assessing these clinical findings, we suspected leptospirosis or typhoid fever and started the administration of ceftriaxone 1 g IV q12h and minocycline 100 mg IV q12h (Figure 2).
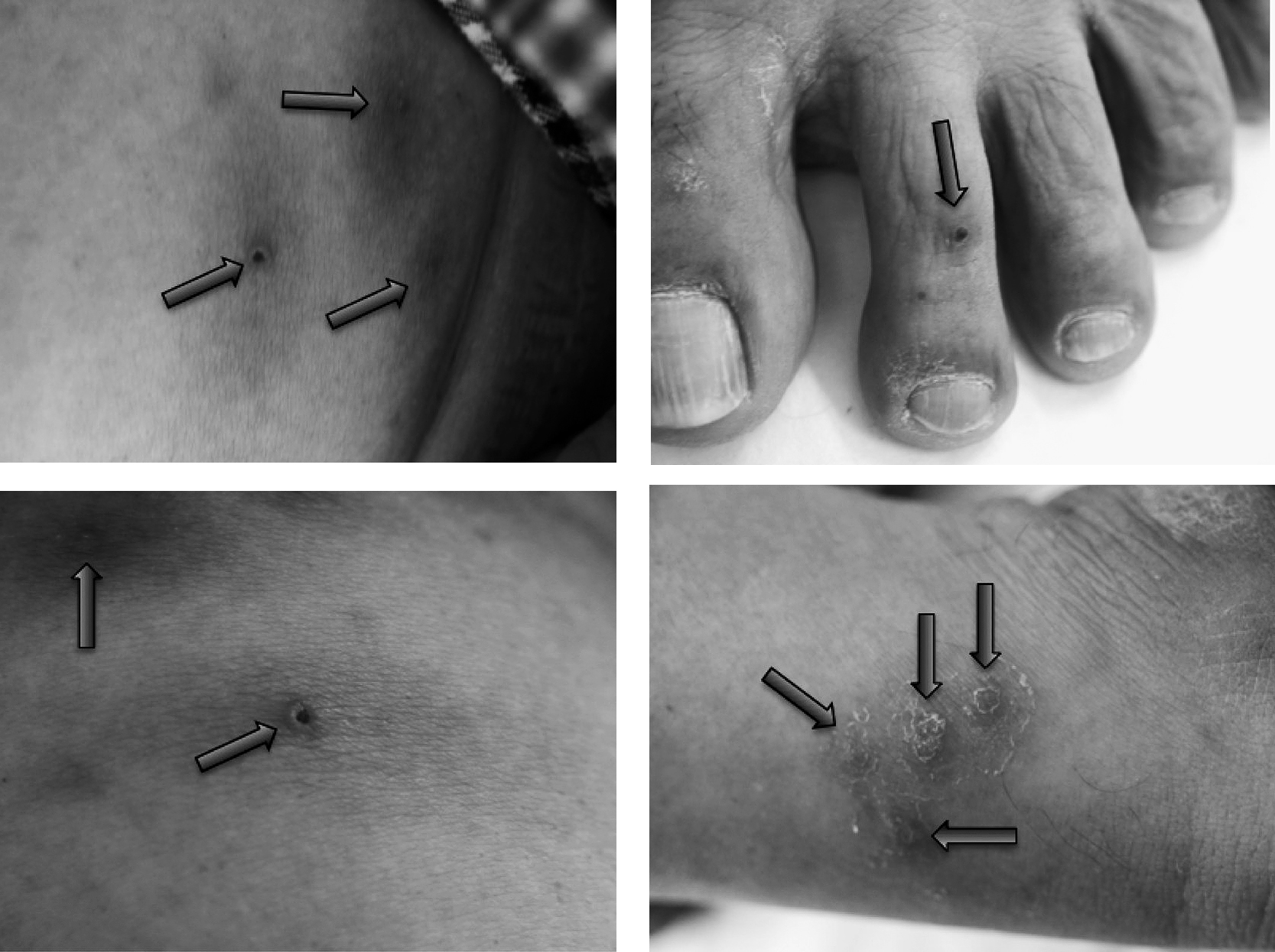
Table 1.
The results of laboratory tests
|
June 2nd |
3rd |
4th |
5th |
6th |
7th |
8th |
9th |
18th |
| CBC: normal range |
| Red Blood Cell (104/µL): /400–570 |
515 |
471 |
451 |
489 |
470 |
491 |
477 |
507 |
459 |
| Hemoblobin (g/dL): 12.9–17.2 |
16.8 |
15.1 |
14.2 |
15.5 |
14.9 |
15.6 |
15.2 |
16.1 |
14.3 |
| Hematocrit (%): 38.2–50.8 |
48.6 |
44.3 |
42.4 |
46.1 |
44.2 |
46 |
44.7 |
47.2 |
43.1 |
| Platelet (104/µL): 14–34 |
17.4 |
18.3 |
17.3 |
18.3 |
16.7 |
20.4 |
21.7 |
26.8 |
25.8 |
| White Blood Cell (/µL): 3,500–8,500 |
12,600 |
12,600 |
5,500 |
4,400 |
5,500 |
6,400 |
5,400 |
5,900 |
5,300 |
| Neutrophil (%): 42–77 |
96.5 |
86.2 |
77 |
44 |
53.5 |
49.5 |
51 |
49.5 |
44.5 |
| Eosinophil (%): 0.5–6 |
0 |
0.7 |
3.5 |
7 |
2.5 |
2 |
2.5 |
2 |
5.7 |
| Basophil (%): 0.1–2 |
0.2 |
0.2 |
0 |
1 |
1 |
2.5 |
1 |
2.5 |
1.7 |
| Lymph (%): 18–49 |
1.9 |
8.1 |
10.5 |
32 |
32 |
41 |
36.5 |
38.5 |
40.1 |
| Mono (%): 3–9 |
0.9 |
4.5 |
7.5 |
16 |
6.5 |
4.5 |
6 |
5 |
7.8 |
| Chemistry: normal range |
| AST (U/L): 13–35 |
59 |
38 |
32 |
26 |
26 |
34 |
30 |
29 |
22 |
| ALT (U/L): 5–35 |
49 |
40 |
35 |
38 |
38 |
48 |
47 |
51 |
24 |
| γ-GTP (U/L): 11–64 |
260 |
267 |
255 |
370 |
292 |
278 |
247 |
247 |
136 |
| UN* (mg/dL): 8–20 |
20 |
33 |
29 |
21 |
19 |
21 |
20 |
20 |
16 |
| CRTN *(mg/dL): 0.6–1 |
1.71 |
3.04 |
2.08 |
1.35 |
1.15 |
1.11 |
1.05 |
1.1 |
1.07 |
| GFR *(mL/min): 90−999 |
33 |
18 |
27 |
43 |
52 |
54 |
57 |
54 |
56 |
| LD* (U/L): 112–230 |
321 |
234 |
216 |
217 |
217 |
260 |
231 |
231 |
191 |
| CK* (U/L): 55–245 |
258 |
274 |
124 |
50 |
37 |
31 |
34 |
35 |
80 |
| T-Bil* (mg/dL): 0.2–1.2 |
2.2 |
1.6 |
0.6 |
0.5 |
— |
— |
0.7 |
— |
0.7 |
| D-Bil* (mg/dL): 0–0.2 |
0.7 |
0.5 |
0.1 |
0.1 |
— |
— |
0.1 |
— |
0.1 |
| CRP (mg/dL): 0–0.3 |
24.789 |
29.381 |
17.904 |
6.983 |
1.694 |
1.033 |
0.617 |
0.418 |
0.082 |
| Urine: normal range |
| Specific Gravity: 1.008–1.03 |
|
1.018 |
1.008 |
1.008 |
1.008 |
1.008 |
1.009 |
|
1.012 |
| White Blood Cell: (–) |
|
(+++) |
(–) |
(–) |
(–) |
(–) |
(–) |
|
(–) |
| Protein: (–) |
|
(++) |
(–) |
(–) |
(–) |
(–) |
(–) |
|
(–) |
| Occult Blood: (–) |
|
(++) |
(+) |
(+/–) |
(–) |
(+) |
|
(–) |
(–) |
| pH: 5–7.4 |
|
5.5 |
5 |
5 |
6 |
5.5 |
5.5 |
|
6 |
*UN: urea nitrogen, CRTN: creatinine, GFR: glomerular filtration rate, LD: lactate dehydrogenase
CK: creatine phosphokinase, T-Bil: total bilirubin, D-Bil: direct bilirubin

Gastrocnemius tenderness, arthralgia and other clinical symptoms had become less severe on the second day of treatment. On the third day of antibiotics administration, urine output improved to more than 3,000 mL/15 hours. The serum level of urea nitrogen and creatinine also improved. The conjunctival jaundice vanished. Body temperature returned to a normal level (36.6°C) on the fourth day (June 6th). Finally, his general condition became better and his appetite returned on the sixth day after intravenous therapy. We switched to oral antibiotics (minocycline 100 mg bid) and continued treatment until June 12th. No adverse reaction was observed during antibiotic therapy. Nine days after admission to the hospital, the patient was in remission and was discharged.
Although no pathogen was detected in the blood, stool or urine samples during the patient’s hospitalization, we collected serum samples on two different days (June 3rd and 18th) for diagnosis. These two serum samples were examined at the National Institute of Infectious Diseases in Japan. Microscopic agglutination test (MAT) using live Leptospira serovar strains revealed a more than four-fold increase in antibody titer in paired serum samples (Table 2). MAT detects sero-group specific antibody. A sero-group of the strain that shows the highest reciprocal MAT titer is considered to be a presumptive infecting sero-group, although it is well known that MAT exhibits a high degree of cross-reaction that occurs between different sero-groups. Therefore, we assume that the infecting sero-group in this case is Australis or Autumnalis. Finally, we arrived at a diagnosis that the patient had suffered from leptospiral infection, Weil’s disease, a severe form of human leptospirosis.
Table 2.
The results of microscopic agglutination test
| Strain |
Antibody titer |
| June 3rd |
June 18th |
| Leptospira borgpetersenii serovar Castellonis |
<40 |
<40 |
| L. borgpetersenii serogroup Javanica |
<40 |
<40 |
| L. borgpetersenii serovar Poi |
10 |
40 |
| L. borgpetersenii serovar Sejroe |
10 |
40 |
| L. borgpetersenii serovar Tarassovi |
<40 |
<40 |
| L interrogans serovar Australis |
<10 |
320 |
| L interrogans serovar Autumnalis |
<10 |
320 |
| L. interrogans serovar Losbanos |
<40 |
<40 |
| L. interrogans serovar Canicola |
<40 |
<40 |
| L. interrogans serovar Copenhageni |
<40 |
<40 |
| L. interrogans serogroup Grippotyphosa |
<40 |
<40 |
| L. interrogans serogroup Hardjo |
<40 |
<40 |
| L. interrogans serovar Hebdomadis |
<40 |
<40 |
| L. interrogans serovar Icterohaemorrhagiae |
<40 |
<40 |
| L. interrogans serovar Wolffi |
<40 |
<40 |
| L. interrogans serovar Pomona |
<40 |
<40 |
| L. interrogans serovar Manilae |
<40 |
<40 |
| L. kirschneri serovar Cynopteri |
<10 |
160 |
| L. noguchii serovar Panama |
<40 |
<40 |
(The National Institute of Infectious Diseases)
Discussion
When a febrile patient who has been to a tropical or subtropical region is encountered, it is important for a differential diagnosis to determine whether he/she is suffering from malarial or non-malarial infection. In this case, the patient was bitten by mosquitoes a number of times without chemoprophylaxis in Vietnam, and he had a high fever ten days after being bitten by mosquitoes. In addition, he permanently resides in a non-malarial endemic region. Therefore, our first suspicion was infection with malaria. Upon examination, the dipstick rapid diagnostic test for Plasmodium species was negative, and the multiple examinations of the Giemsa-stained peripheral-blood thin smear (pH 7.2) did not show any infected erythrocytes at all. Hence we assumed that the possibility of malaria infection in this patient was extremely low. In view of the renal function disorder and his water contact history in Vietnam, we started treatment for suspected leptospirosis. Finally, he was diagnosed as leptospirosis by the detection of specific antibodies against serovar Australis and Autumnalis strains. L. fainei serovar Hurstbridge is the predominant form of leptospirosis in cases of acute jaundice disease identified from Indonesia, Lao P.D.R., and Vietnam in a previous report [4]. This report indicated that the predominant serovars are Bataviae, Semaranga, and Hurstbridge in Vietnam. A large number of serovars has been recognized recently in genus Leptospira, and various serovars have been reported in various countries and areas. This should be carefully noted when considering the possibility of leptospiral infection.
The patient in this case revealed myalgia, jaundice, proteinuria and petechia at the early clinical stage. Therefore, the case is consistent with the definition proposed by Dr. Weil in 1886, namely “Weil’s disease”, a severe human leptospirosis. Although almost 90% of leptospiral infection cases exhibit a febrile self-limited illness, 10% may develop to Weil’s disease [5]. Renal failure in Weil’s disease is a common complication, and the pathological condition is primary ischemia with subsequent tissue hypoxia and renal tubular damage. The clinical condition is generally reversible, and in this case renal function also recovered quickly after administration of antibiotics with hydration. It was not necessary to use hemodialysis in the end. We could not rule out the possibility of typhoid infection. The patient revealed a severe general status, and ceftriaxone and minocycline were selected to be administered simultaneously for treatment. Some previous case reports indicated that infection with serovar Icterohaemorrhagiae was related to severe human leptospirosis [6–9]. Although the microscopic agglutination test showed no significant increase in antibody titer for serovar Icterohaemorrhagiae in this patient’s paired serum, the present case report indicated that any serovar infection could cause the severe condition in the host.
With the expansion of globalization, we should concurrently pay attention to the globalization of infectious diseases. Transportation has become more and more extensive in recent years, allowing pathogens to move long distances using modern facilities. The variety of human activities has also become wide-ranging. Leptospirosis is endemic in tropical and subtropical areas, urban in urban as well as rural areas, and it has been traditionally recognized as an occupational risk among professionals in contact with water that could be contaminated with animal urine [10]. Generally speaking, the occupational risk groups are sewer workers, farmers, abattoir workers, fish farmers, veterinarians, hunters, biologists, zoologists, and cultural anthropologists. Looking at the current situation of leptospirosis, however, the important risk groups for leptospirosis may include adventure travelers and athletes participating in fresh water sports [11]. In the previous reports of imported cases in Japan, the patients became infected during vacations. The patient discussed in the present study is a scientific researcher. Therefore, adequate attention should be paid to various conditions. The mountain areas where this patient traveled did not have any facilities for bathing or obtaining potable water. He had to make use of nearby rivers. This situation might be one of the risk factors for leptospiral infection. Leisure activities in the endemic areas increase the risk of leptospiral infection as well. We should also keep in mind infection due to occupational risks.
Vietnam is a popular tourist destination for Japanese travelers and also one of the popular countries for Japanese expatriates who work overseas. There are more than 9,300 Japanese residents in Vietnam [12]. Twelve years have passed since suggestions for Japanese travelers were published [13]. Regardless of purpose or activity, those who travel to Vietnam and other tropical/subtropical regions must be aware of the risk of leptospiral infection. They can consult travel medicine professionals for information on both the obvious and potential health risks associated with traveling abroad (even though leptospire infection can also occur in Japan). High-risk travelers can take preventive measures, such as vaccination and chemoprophylaxis. Meanwhile, travel medicine professionals should conduct educational activities aimed at informing patients of health risks related to travel abroad.
Acknowledgments
We are deeply grateful to Dr. Nobuo Koizumi, Department of Bacteriology, the National Institute of Infectious Diseases, for examining the microscopic agglutination tests used for the definite diagnosis. It was a great help for both diagnosis and treatment.
References
- 1 Amilasan AS, Ujiie M, Suzuki M, Salva E, Belo MC, Koizumi N, Yoshimatsu K, Schmidt WP, Marte S, Dimaano EM, Villarama JB, Ariyoshi K. Outbreak of Leptospirosis after Flood, the Philippines, 2009. Emerg Infect Dis 2012; 18(1): 91–94.
- 2 Koizumi N, Watanabe H. Current Knowledge of Leptospira and Leptospirosis. Modern Media 2006; 52(10): 290–306.
- 3 Journal of Health and Welfare Statistics, Extra Edition, 2009; 56(16): 24–25
- 4 Laras K, Cao BV, Bounlu K, Nguyen TK, Olson JG, Thongchanh S, Tran NV, Hoang KL, Punjabi N, Ha BK, Ung SA, Insisiengmay S, Watts DM, Beecham HJ, Corwin AL. The Importance of Leptospirosis in Southeast Asia. American Journal of Tropical Medicine and Hygiene 2002; 67(3): 278–286.
- 5 Antony SJ. Leptospirosis—An Emerging Pathogen in Travel Medicine: A Review of its Clinical Manifestations and Management. J Travel Med 1996; 3: 113–118.
- 6 Arcilla MS, Wismans PJ, van Beek-Nieuwland Y, van Genderen PJ. Severe Leptospirosis in a Dutch Traveller Returning from the Dominican Republic, October 2011. Euro Surveill 2012; 17(13): pii=20134, 1–2.
- 7 Roczek A, Forster C, Raschel H, Hörmansdorfer S, Bogner KH, Hafner-Marx A, Lepper H, Dobler G, Büttner M, Sing A. Severe Course of Rat Bite-Associated Weil’s Disease in a Patient Diagnosed with a New Leptospira-Specific Real-Time Quantitative LUX-PCR. J Med Microbiol 2008; 57: 658–663.
- 8 Clavel M, Lheritier G, Weinbreck N, Guerlin A, Dugard A, Denes E, Vignon P. Leptospirosis: An Unusual Cause of ARDS. Crit Care Res Pract 2010: Article ID 408365, 1–3.
- 9 Masuda K, Uehara Y, Ono H, Furukawa K. A Case of Severe Leptospirosis Infection (Weil’s Disease) in Tokyo. Kansenshogaku Zasshi 2010; 84(1): 59–64.
- 10 Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM; Peru-United States Leptospirosis Consortium. Leptospirosis: A Zoonotic Disease of Global Importance. Lancet Infect Dis 2003; 3: 757–771.
- 11 Pavli A, Maltezou HC. Travel-Acquired Leptospirosis. J Travel Med 2008; 15(6): 447–453.
- 12 Annual Report of Statistics on Japanese Nationals Overseas 2012. Consular Policy Division, Consular Affairs Bureau, Minister of Foreign Affairs, Japan.
- 13 Basnyat B, Pokhrel G, Cohen Y. The Japanese Need Travel Vaccinations. J Travel Med 2000; 7(1): 37.



