2014 年 42 巻 3 号 p. 121-126
2014 年 42 巻 3 号 p. 121-126
BACKGROUND: Ethics is one of the main pillars in the development of science. We performed a JoinPoint regression analysis to analyze the trends of ethical issue research over the past half century. The question is whether ethical issues are neglected despite their importance in modern research.
METHOD: PubMed electronic library was used to retrieve publications of all fields and ethical issues. JoinPoint regression analysis was used to identify the significant time trends of publications of all fields and ethical issues, as well as the proportion of publications on ethical issues to all fields over the past half century. Annual percent changes (APC) were computed with their 95% confidence intervals, and a p-value < 0.05 was considered statistically significant.
RESULTS: We found that publications of ethical issues increased during the period of 1965–1996 but slightly fell in recent years (from 1996 to 2013). When comparing the absolute number of ethics related articles (APEI) to all publications of all fields (APAF) on PubMed, the results showed that the proportion of APEI to APAF statistically increased during the periods of 1965–1974, 1974–1986, and 1986–1993, with APCs of 11.0, 2.1, and 8.8, respectively. However, the trend has gradually dropped since 1993 and shown a marked decrease from 2002 to 2013 with an annual percent change of –7.4%.
CONCLUSIONS: Scientific productivity in ethical issues research on over the past half century rapidly increased during the first 30-year period but has recently been in decline. Since ethics is an important aspect of scientific research, we suggest that greater attention is needed in order to emphasize the role of ethics in modern research.
Ethics is “concerned with moral principles, values and standards of conduct” [1] and has become indispensable for conducting a sound research, both qualitative and quantitative. Many codes and principles of ethics have been established to ensure the safety and privacy of research participants. In the 5th century, Formula Comitis Archiatrorum, the earliest known code of medical ethics, was written [2]. The Ethical Principles of Psychologists and the Code of Conduct of the American Psychological Association, published in 2002, are statements on ethical issues for psychologists [3]. A Code of Ethics for Public Health was also released by the American Journal of Public Health in 2002, identifying the “distinctive elements of public health and the ethical principles that follow from or respond to those elements” [4]. These codes and principles aim to provide basic guidelines for researchers to conduct research without harming participants.
Despite the numerous ethical codes and principles published worldwide, ethical issues in research have become even more complicated [5–10]. Many studies, especially those that involve human subjects, have to deal with ethical challenges, which might slow or even stop the reseach [11, 12]. Consequently, more research involving ethical issues, particularly in clinical trials, needs to be conducted in this century. “The impact of clinical trials not only extends to the individual patient by establishing a broader selection of effective therapies, but also to society as a whole” [13]. The more ethical issues arise in clinical trials, the more research is needed to address these issues.
In this study, we looked at whether ethical issues have recently been neglected despite their its importance in modern science. The idea was derived from our observation that while publications of all fields are increasing considerably, the number of publications of ethical issues has not shown a corresponding trend. Through this study, we aim to confirm this observation and thus to promote more attention regarding ethical issues in research.
According to the PubMed MeSH Database, MeSH terms are the main tool for searching life sciences and biomedical references. Many scientific articles on bibliometrics have used MeSH terms as the principle method to collect raw data [14–17]. We performed a search strategy on the PubMed electronic library (http://www.ncbi.nlm.nih.gov/PubMed) and retrieved all publications of all fields (APAF) each year using the search term ‘“all”[sb]’. All publications of ethical issue (APEI) were found using the MeSH term ‘“Ethics”[Mesh]’. There was no restriction on text availability, publication dates, or species.
The period of the retrieved APAF and APEI results were 1809–2015 and 1945–2014, respectively. The adoption of the Declaration of Helsinki (1964) heralded a new era of ethics. Therefore, we confinded our publication trend analysis to the period from 1965 to 2013 (Table 1). Thereafter, we also analyzed the relative publication numbers (proportion of APEI and APAF) to determine whether research related to ethical issues has been neglected.
| Year | APAF# | APEI‡ | Proportion of APEI and APAF |
|---|---|---|---|
| 1965 | 176545 | 233 | 0.00132 |
| 1966 | 179784 | 272 | 0.00151 |
| 1967 | 191644 | 365 | 0.00190 |
| 1968 | 207672 | 586 | 0.00282 |
| 1969 | 214858 | 489 | 0.00228 |
| 1970 | 219056 | 532 | 0.00243 |
| 1971 | 223035 | 524 | 0.00235 |
| 1972 | 227189 | 565 | 0.00249 |
| 1973 | 230550 | 916 | 0.00397 |
| 1974 | 234496 | 934 | 0.00398 |
| 1975 | 248179 | 1037 | 0.00418 |
| 1976 | 254219 | 902 | 0.00355 |
| 1977 | 261103 | 1063 | 0.00407 |
| 1978 | 271465 | 1104 | 0.00407 |
| 1979 | 280661 | 1141 | 0.00407 |
| 1980 | 279229 | 1185 | 0.00424 |
| 1981 | 281966 | 1159 | 0.00411 |
| 1982 | 294004 | 1196 | 0.00407 |
| 1983 | 307830 | 1438 | 0.00467 |
| 1984 | 316723 | 1500 | 0.00474 |
| 1985 | 333701 | 1650 | 0.00494 |
| 1986 | 347887 | 1738 | 0.00500 |
| 1987 | 365805 | 1895 | 0.00518 |
| 1988 | 384104 | 2103 | 0.00548 |
| 1989 | 400624 | 2524 | 0.00630 |
| 1990 | 408418 | 2867 | 0.00702 |
| 1991 | 409907 | 2935 | 0.00716 |
| 1992 | 415398 | 3278 | 0.00789 |
| 1993 | 423827 | 3685 | 0.00869 |
| 1994 | 434932 | 3890 | 0.00894 |
| 1995 | 446170 | 3709 | 0.00831 |
| 1996 | 456064 | 3844 | 0.00843 |
| 1997 | 453427 | 4102 | 0.00905 |
| 1998 | 470953 | 4084 | 0.00867 |
| 1999 | 490526 | 4137 | 0.00843 |
| 2000 | 528244 | 4341 | 0.00822 |
| 2001 | 540799 | 4888 | 0.00904 |
| 2002 | 556083 | 4825 | 0.00868 |
| 2003 | 581815 | 4069 | 0.00699 |
| 2004 | 619075 | 4344 | 0.00702 |
| 2005 | 654226 | 4283 | 0.00655 |
| 2006 | 681379 | 4275 | 0.00627 |
| 2007 | 706692 | 3976 | 0.00563 |
| 2008 | 746040 | 4073 | 0.00546 |
| 2009 | 778762 | 4105 | 0.00527 |
| 2010 | 816316 | 4150 | 0.00508 |
| 2011 | 866091 | 3783 | 0.00437 |
| 2012 | 926333 | 3823 | 0.00413 |
| 2013 | 1005418 | 3076 | 0.00306 |
#APAF: All publications of all fields
‡APEI: All publications of ethical issues
The annual percent change (APC) is used as a parameter to measure trends in diseases, mortality rate, and number of publications over time in medical research [18–21]. It is a simple and effective tool to compare the rate of changes at different time intervals. The APC from year t to year (t + 1) can be described by the following equation:
ln(R) is the natural logarithm of the rate in year t [22] calculated by the following equation:
All statistical analyses were performed using the help of JoinPoint Regression Program version 4.1.0 [23]. The details of this model is established by Kim HJ et al [24]. Briefly, we assigned the year of publication as an independent variable and the number of publications and relative publication number as dependent variables. APAF and APEI were set at “count” type while the proportion of APEI and APAF were set at “proportion”. Also, log transformation was used for all analyses. At weighted least squares, we selected the “Constant Variance” option. Thus, in the “advanced” tab, we chose “grid-search” option with “fit an uncorrelated errors model” and used “Permutation test” option to evaluate the accurate number of join points [25].
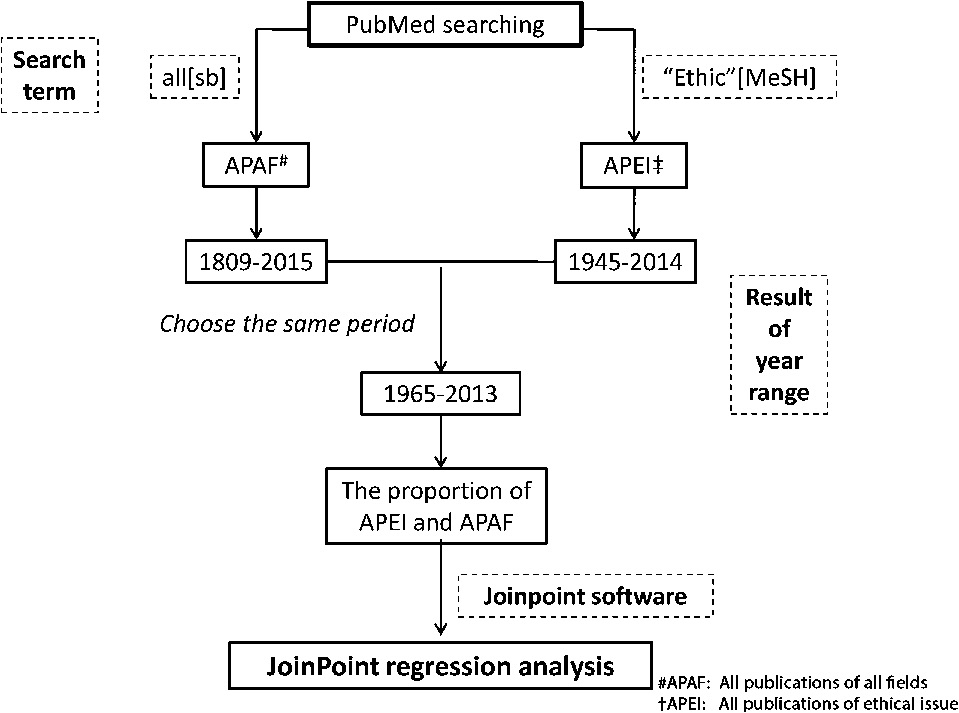
The workflow search and data analysis.
Regarding the range of years, a P-value smaller than 0.05 was considered a significant change in trend.
As expected, the number of publications, in general, showed an exponential increase over the past half century (Table 2). APAF steadily and continuously increased during the 1965–2013 period (Fig. 2A), and the highest APC was seen during the last three years (APC = 8.25%, 95% CI: 4.3–12.3). Similarly, the APEI showed a significant increasing trend from 1965 to 1995 (Fig. 2B). However, a decline in the trend of APEI was seen from 1996 to 2013 (APC = −0.99%, 95% CI: −1.7–−0.3, P < 0.05).
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | Trend 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC** | Years | APC** | Years | APC** | Years | APC** | Years | APC** | Years | APC** | |
| #APAF | 1965–1968 | 6.03^ | 1968–1982 | 2.57^ | 1982–1989 | 4.38^ | 1989–1998 | 1.84^ | 1998–2011 | 4.75^ | 2011–2013 | 8.25^ |
| ‡APEI | 1965–1968 | 34.46^ | 1968–1971 | –1.08 | 1971–1974 | 23.94^ | 1974–1981 | 2.61 | 1981–1996 | 9.40^ | 1996–2013 | –0.99^ |
| APEI/APAF | 1965–1974 | 10.97^ | 1974–1986 | 2.07^ | 1986–1993 | 8.84^ | 1993–2002 | –0.35 | 2002–2013 | –7.35^ | ||
**APC = Annual percent changes calculated by Joinpoint regression analysis
^APC is significantly different from zero when P < 0.05
#APAF: All publications of all fields
‡APEI: All publications of ethical issues

Trend of publication numbers in all fields (APAF) and ethical issues (APEI) over the past half century. The annual percent change (APC) was computed using Joinpoint regression analysis. Symbol “^” indicates a significant difference from zero at P value < 0.05.
The proportion of APEI to total publications was relatively low (mean = 0.54%) with two opposing trends. The first trend showed an uninterrupted rise from 1965 to 1993, splitting into three time periods with different rates: 1965–1974 (APC = 10.97%, 95% CI: 8.2–13.8), 1974–1986 (APC = 2.07%, 95% CI: 0.2–4.0), and 1986–1993 (APC = 8.84%, 95% CI: 3.8–14.1). The second trend showed a significant decrease over the past twenty-one years with two different rates: 1993–2002 (APC = −0.35%, 95% CI: −3.4–2.7), and 2002–2013 (APC = −7.35%, 95% CI: −9.1–−5.6) (Fig. 3).

Trend of the proportion of APEI to APAF over the past half century. The annual percent change (APC) was computed using Joinpoint regression analysis. Symbol “^” indicates a significant difference from zero at P value < 0.05.
In August 1947, the “Nuremberg Code” was adopted under the title of “permissible medical experiments” “in order to satisfy moral, ethical and legal concepts” for experiments involving human subjects [26]. Subsequently, many declarations regarding ethical issues in research were published, such as the Declaration of Geneva (1948), World Medical Association (WMA) International Code of Medical Ethics (1949), Wilson Memo (1953), WMA Principles for Those in Research and Experimentation (1954), and Declaration of Helsinki (1964) [27–29]. These declarations put proper emphasis on ethical issues, thus encouraging researchers to focus more attention on the subject. This explains the upward trend in the absolute number and relative number of publications related to ethical issues in the twentieth century (Fig. 2B, and 3). However, the decreasing proportion of publications in ethical issues compared to all publications over the last twenty-one years is likely due to the saturated level of research regarding ethical issues or an inadequate interest during the period.
This study aims to promote interest among researchers in ethical issues and their application in conducting clinical trials of ethics. This will eventually help clinical practitioners to adopt a more appropriate attitude in their career. The relationship between doctors and patients, and between researchers and participants, can also be expected to improve when more research regarding ethics is conducted [30–32]. A good example is informed consent. There is no doubt that informed consent has become a vital role in conducting clinical trials. However, the comprehension of participants on the process of informed consent in research remains poor [33]. If researchers do not pay proper attention to participants’ understanding regarding the informed consent process, resistance or disagreement might occur later. Nevertheless, only a few systematic reviews and meta-analyses regarding informed consent have been conducted to date. This is a considerable deficiency and shows that there are still ethical issues requiring the attention of researchers.
This study analyzes ethical issues in all subjects and so it does not provide a specific evaluation of any field. The database source of the research also completely depends on the selection and categorization of publications on PubMed, which may cause possible bias. Another limitation is that our study did not limit the fields of publications during the analyzed period, so the increase of ethics publications might be due to the appearance of new fields involving ethical issues rather than the increase of publications in each field.
Our results showed that the amount of research on ethical issues has been decreasing in recent years. This trend suggests that ethical issues are a grave concern in recent research despite theirs vital importance in the research process of any field as mentioned above. Ethics has a more and more significant role to play in modern research in order to ensure legality and safety, especially in the twentieth century—the splendid age of scientific research [34–37]. We urge that researchers to be more concerned with conducting ethics-related research in the coming years, so that ethics can gain its rightful place in modern research in all fields of life sciences, especially those involving human subjects.
We thank Ms Junjira Laothavorn J.D., MSc (Albany Law School, Albany, New York, USA) for her help in editing the manuscript.
NPL and NTHT have been awarded scholarships from the Vietnam Student Development Fund (VNSDF; www.vnsdf.org). The funder had no role in the study design, data collection and analysis, preparation of the manuscript, or decision to publish.