論文ID: 2023-007
論文ID: 2023-007
The role of messenger (m) RNA vaccines in the outbreak of severe acute respiratory syndrome-related coronavirus (SARS-CoV-2) has been very significant. It is necessary to consider whether there are any mRNA-specific aspects to be taken into account regarding the vaccines’ efficacy and safety, as well as the design and quality control of mRNA, with an eye toward the development of mRNA vaccines for infectious diseases other than SARS-CoV-2. This review discusses regulatory requirements for assessing the quality, efficacy, and safety of developing mRNA vaccines.
The role of messenger (m) RNA vaccines in the 2020 outbreak of severe acute respiratory syndrome-related coronavirus (SARS-CoV-2) has been significant. mRNA vaccines were the earliest SARS-CoV-2 vaccines to be approved, and their efficacy in preventing the onset of coronavirus disease (COVID)-19 has exceeded 90% [1, 2], with high efficacy in preventing severe disease [2, 3]. The mRNA vaccines have been commercialized to tackle the COVID-19 pandemic, although this modality has not been used for other vaccines.
The high efficacy of mRNA vaccines is due not only to the high antibody titer induced by the vaccines but also to the high-level induction of both humoral and cellular immunity [4] by the expression of the antigen in cells. Although this has not been established, it has been suggested that innate immune activation provided by mRNA vaccines may also contribute to their high efficacy [5], as depicted in Fig. 1 The application of mRNA vaccines to other infectious diseases, such as seasonal influenza, has been attempted; however, the immune responses to these vaccines have not always been as drastic as those to SARS-CoV-2, and the vaccines have not shown superiority over conventional hemagglutinin split vaccines at this stage [6].

Induction of an antiviral immune response by messenger ribonucleic acid (mRNA) administration. Antigen translated from mRNA taken into cells is presented to major histocompatibility complex (MHC)-II as an exogenous antigen, and the antigen presented to MHC-II induces antibody production (humoral immunity) through the activation of helper T cells. In addition, some antigen molecules translated from mRNA are processed by proteasomes and cross-presented by MHC-I to induce the activation of cellular immunity via activation of cytotoxic T lymphocytes (CTLs).
Evaluations of the quality, efficacy, and safety of mRNA vaccines have had to be conducted in the absence of sufficient knowledge and accumulated experience; therefore, it is necessary to consider the principles of these evaluations with a view toward the future development of vaccines for other infectious diseases. In particular, it is necessary to consider whether there are any mRNA-specific aspects to be taken into account regarding the vaccines’ efficacy and safety, as well as the design and quality control of mRNA, with an eye toward the development of mRNA vaccines for infectious diseases other than SARS-CoV-2. mRNA products that have proliferative properties in administered cells have also been developed [7, 8]. This review discusses regulatory requirements for assessing the quality, efficacy, and safety of the development of mRNA vaccines. However, it is assumed that the concept of non-clinical and clinical studies in vaccine development should be based on relevant guidelines.
The design of mRNA vaccines for viral infections is based on the mechanism of infection of the target virus within cells. In most designs, sequences encoding viral surface proteins that bind to cell receptors are selected for viral infection. However, it is generally known that (i) through viral infection, multiple cellular proteins are often involved in viral binding to the cell for the introduction of the viral genome into the cell, and (ii) multiple viral proteins are involved in the response to the infection process and are thus involved in the expression of molecules involved in the infection process other than viral proteins that bind to cell receptors. Therefore, in the design of vaccines, it is very important to determine the types of proteins that are expressed other than the viral proteins that bind to cell receptors and are involved in the infection process [9, 10]. In addition, the form of the expressed target viral antigen, that is, as a secretory protein, membrane protein, or particle formation, is closely related to the mRNA vaccine development strategy. When the application for the vaccine’s approval by a pharmaceutical regulatory is being processed, it will also be necessary to explain the product design, including the reason (s) for selecting the protein to be expressed and its validity from the viewpoint of preventing the target infectious disease.
The basic structure of a non-proliferative mRNA vaccine consists of a 5′-Cap, a 5′ untranslated region upstream of the target gene, an open reading frame that encodes the antigen, a 3′ untranslated region downstream of the target gene, and a poly (A) tail (Fig. 2). The nucleosides that make up mRNA activate innate immunity [11,12,13]. Using modified nucleosides (such as pseudouridine) instead of unmodified nucleosides to suppress the activation of innate immunity is an important factor in mRNA designs for a better risk-benefit balance [14, 15]. In addition, double-stranded (ds) RNA and the conformation of the product can activate innate immunity. For example, dsRNA is known as a promoter of type I interferon production through the TLR3, RIG-I, and MDA5 pathways [16].
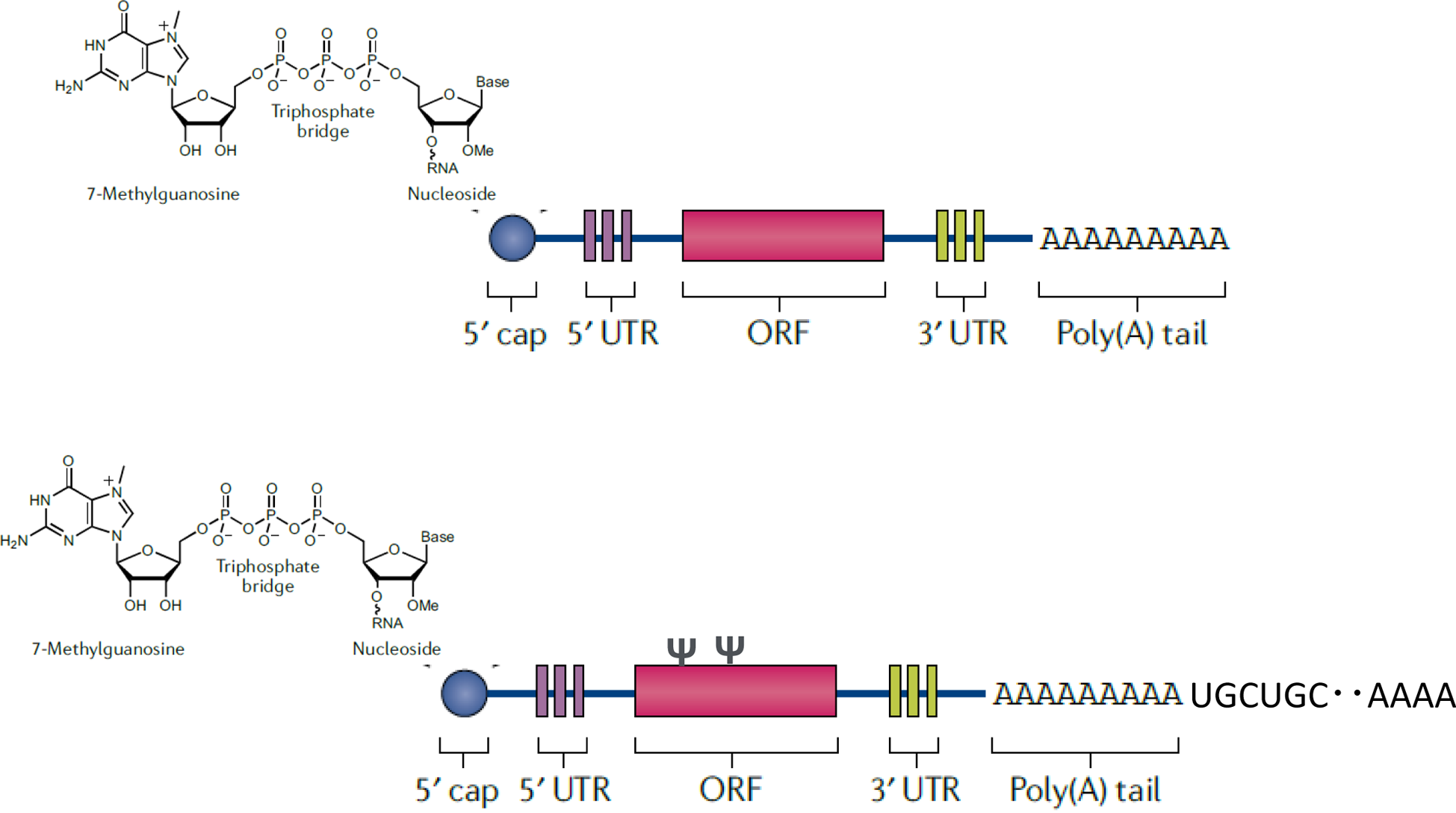
Basic structure of an messenger ribonucleic acid (mRNA) vaccine. The mRNA, the drug substance of the mRNA vaccine, has undergone multiple modifications, including the substitution of nucleosides from uridine to pseudouridine and the insertion of UGC repeats into the poly (A) tail. 5’ UTR: 5’ untranslated region; 3 ‘UTR: 3’ untranslated region.
Increasing G:C mRNA content has been reported to contribute to mRNA stabilization during protein translation and expression both in vitro and in vivo [17, 18]. Using more frequent codons in human cells may also lead to an increased expression of target proteins. How mRNA sequences are designed may have a significant impact on the persistence of antigen expression and induction of immunity. Conversely, modifications of an mRNA sequence can lead to unexpected structural changes that may significantly affect sequence translation [19].
The design used for constructing mRNA and its manufacturing process to achieve efficient production also provides important information for the quality control of mRNA vaccines. For example, it is important to develop appropriate control strategies for a drug substance and product. This strategy includes the preparation of template DNA used in the in vitro transcription process or the purification of mRNA from the raw materials used in the transcription process. In addition, if an mRNA sequence or nucleoside is modified during the development phase, evaluating how antigen expression is affected by such changes is useful to justify the appropriateness of those modifications.
The design of mRNA constructs and the manufacturing process for efficient production of mRNA are very important for understanding the properties and quality attributes of mRNA vaccines used as pharmaceuticals.
A method for self-amplifying mRNA has been developed for the manufacture of pharmaceuticals. This method amplifies the expression of target mRNA in human cells by inserting an alpha virus RNA-dependent RNA polymerase genome into the region upstream of the target antigen in the mRNA sequence [20]. When this self-amplifying RNA system is used, the innate immune response is expected to be triggered by the generated RNA, which may induce a strong adjuvant effect of the vaccine.
When producing mRNA vaccines, the in vitro transcription reaction and, in some cases, the chemical addition reaction (i.e., adding a 5′-Cap structure or poly (A)) are combined based on the design of the target mRNA. Since the composition of impurities differs depending on how these reactions are used, it is important to evaluate the quality of mRNA while considering the manufacturing process. mRNA production begins with in vitro transcription. The raw materials used in the manufacturing process, such as the template DNA, RNA polymerase, substrate nucleic acids, enzyme reaction solution, 5′-Capping enzymes and their substrate, and poly (A) polymerase, should be sufficiently removed after the reaction. Thus, it is important to determine the residual amounts of these impurities during the manufacturing of the final products.
The poly (A) sequence plays an important role in mRNA translation and intracellular stability [21], and its chain length must be ≥100 mer [10]. To add the optimal length of poly (A) [25] to mRNA, it is either transcribed directly from a DNA template encoding the antigen and a poly (A) or added poly (A) sequence using poly (A) polymerase after the transcription reaction to the end of the mRNA sequence. It is also known that expressing a long poly (A) in a plasmid causes instability; therefore, UGC sequences were inserted into the poly (A) chain to increase its stability (Fig. 2) [26, 27].
One of the most important processes in mRNA vaccine manufacturing is the production of the template for the in vitro transcription, which is obtained using Escherichia coli plasmids. Generally, purified plasmids produced in E. coli are linearized and used as templates for in vitro transcription. In some cases, one or more plasmids (which are also combined with another plasmid) or target sequences amplified by polymerase chain reaction (PCR) are used to manufacture templates. The mRNA is transcribed from these templates in an in vitro transcription reaction using RNA polymerase. Because the plasmids are amplified by E. coli, it is important to control impurities derived from the host cells of E. coli (such as proteins and DNA) in the production of templates during the extraction and purification processes. If the templates are contaminated with fragmented DNA from the plasmid or the circular plasmid, incomplete mRNA transcription may be contaminated in the final products. Thus, it is important to evaluate incomplete mRNA transcription as potential product-related impurities.
The template used for the in vitro transcription reaction can be regarded as the starting material for mRNA production. However, its quality attributes, including purity, may affect the quality of the mRNA produced, and quality control is very important for the production of mRNA vaccines of consistent quality. Thus, strict acceptance specifications and appropriate process control for the plasmids, which are the raw materials for templates, are required. It is also necessary to consider the possibility that the amount of each nucleoside triphosphate to be added may be a critical process parameter in the in vitro transcription reactions [29].
During transcription, mRNA is synthesized by a DNA-dependent RNA polymerase, such as T7, SP6, or T3, using ATP, GTP, CTP, UTP, or their modified nucleoside triphosphates as substrates. It is important for the poly (A) sequences of mRNA to have sufficient lengths, but it is a challenge to control the strand length of poly (A) in the mRNA manufacturing process; thus, it is important to evaluate the heterogeneity of poly (A) lengths for the quality control of mRNA. In the transcription process, a 5′-Cap structure [21] is added to the 5′ end of the mRNA, and this addition is important for the stability of mRNA, providing a shield to the 5′ end of the mRNA and resistance to exonucleases. For this purpose, the 5′-Cap can be added by various methods, such as the addition of a vaccinia virus-derived capping enzyme [22] during the transcription reaction, the addition of a synthetic Cap after the transcription reaction, or the incorporation of an anti-reverse Cap analog [23, 24]. In the process of these reactions, the products may contain incomplete mRNA to which Cap is not added, and the efficiency of the reaction to the addition of a 5′-Cap is one of the key issues to be checked when the quality of mRNA vaccines is evaluated.
After in vitro transcription, the target mRNA is purified. mRNA, as a drug substance, is first treated by (i) degradation of the DNA used as the template, (ii) removal of impurities using tangential flow filtration or other purification processes, and (iii) concentration of the mRNA. Purified drug substance (DS) must be prepared without RNases to ensure mRNA stability.
It is also expected that the DNA used as a template is degraded by DNase after the transcription reaction and fully removed by the mRNA purification process. The World Health Organization (WHO) has established the criteria for acceptable amounts of host cell-derived DNA in conventional vaccines, which are classified into two groups: cancer cell-derived DNA and non-cancer cell-derived DNA. This is based on the assumption that there is a certain degree of cellular DNA uptake following local vaccine administration [28]. These criteria also mention the length of the cell-derived DNA. The mRNA in mRNA vaccines is usually encapsulated in lipid nanoparticles (LNPs), and formulation technology is used to ensure that the mRNA is actively taken up by the cells. Therefore, when mRNA vaccines are administered, DNA contaminating the target mRNA as an impurity is actively delivered into the cells together with the mRNA. Therefore, it is necessary to determine whether the conventional WHO concept of DNA persistence is sufficient. It is necessary to explain the reason (s) for the persistence of DNA, including not only the amount of residual DNA but also its strand length.
Process control is important for the control of contamination and removal of impurities that affect the quality of an mRNA DS, and appropriate process parameters should be established in the purification process related to the removal of impurities. For impurities derived from a manufacturing process that can be sufficiently removed in a specific process, it may be more reasonable to ensure quality through in-process control testing instead of controlling the final product.
During the formulation process, mRNA is encapsulated in lipid-nano-particles (LNPs) to ensure mRNA stabilization, a drug delivery system for delivery to target cells, and intracellular delivery. The LNP encapsulation process is the most important step in drug formulation, and it is important to evaluate process-related impurities, such as the encapsulation ratio after LNP encapsulation, particle size, content of intact mRNA, and characteristics and content of degraded mRNA.
It is also necessary to evaluate the biological activity of a drug product, its ability to express the target antigen using the formulation, and the characteristics of the expressed antigen to explain the validity of the formulation process to a regulatory agency.
To confirm that mRNA with the target quality attributes has been prepared, the physical and chemical properties and biological activity of the DS must be determined. It is necessary to identify the types of process-related and product-related impurities present and estimate their amounts in the product. The basic sequence structure of the non-amplified mRNA is shown in Fig. 2 The mRNA primary sequence is often confirmed by oligonucleotide mapping analysis using liquid chromatography-tandem mass spectrometry. Ultra-performance liquid chromatography/ultraviolet-mass spectrometry can also be used to confirm the integrity of mRNA based on its strand length.
For example, capillary gel electrophoresis or reversed-phase-high-performance liquid chromatography (RP-HPLC) can be used to clarify the ratio of complete to incomplete mRNA contained in a DS. Quantitative PCR and digital PCR were used to estimate the amounts of mRNA and incomplete mRNA. Digital PCR was used to estimate the ratio of complete to incomplete mRNA. The heterogeneity of the added poly (A) chains may be difficult to fully analyze using liquid chromatography-mass spectrometry, and the ratio of their presence (heterogeneity) should be clarified using an analytical method such as RP-HPLC. Heterogeneity may differ depending on whether the poly (A) addition reaction is performed after the in vitro transcription reaction and/or whether the poly (T) sequence is used as a template.
RP-HPLC is a useful tool for analyzing product-related impurities, such as mRNAs that have no Cap structure added, incomplete transcripts, and mRNAs with insufficient poly (A) chain length. Other product-related impurities may be required to analyze the dsRNA content. Because dsRNA is considered a potent activator of innate immunity, it is often analyzed using immunoblotting or other methods.
It is known that mRNAs have sequence-dependent secondary and higher-order structures, information on which can be obtained using circular dichroism spectroscopy, nuclear magnetic resonance spectroscopy, and analysis of thermodynamic parameters using differential calorimetry. Even if these methods are applied, the precise extent to which the higher-order structure of the target mRNA is involved in its biological characteristics, such as activity and stability, remains unknown. These methods may be useful for stability tests and assessments of product comparability before and after changes [29].
When confirming (i) the ability of mRNA to express proteins, including target antigens, as the biological activity of the mRNA and (ii) the characteristics of the expressed proteins, antigen proteins can be expressed by transfection of mRNA into appropriate model cells in vitro, and the proteins can be analyzed by western blotting. However, it is difficult to analyze the mode of existence of proteins expressed from mRNA using western blotting alone. For example, if the expressed protein is designed to be distributed on the cell membrane surface, characterization of the protein may not be sufficient unless the distribution of the protein in the expressing cell is analyzed, and the expressed protein is confirmed to be on the cell membrane surface. In other cases, it may be appropriate to reveal biological activity by performing a quantitative cellular immunological analysis of the expression of the target protein in mRNA-treated cells. It is also possible to demonstrate the usefulness of a protein expressed by mRNA as a target antigen by analyzing the reactivity of the target protein expressed in cells using antibodies against the target viral antigen contained in the plasma of convalescent patients [30, 31].
Potential impurities from the manufacturing process include template DNA used for in vitro transcription reactions, RNA polymerase, DNase used to degrade DNA after a transcription reaction, residual host cell proteins, cellular DNA, and endotoxins in the templates derived from E. coli. It is important to evaluate not only the amount of DNA present but also the strand length of the remaining DNA. Measuring the amount of long-stranded DNA is particularly important.
For enzymatic elongation of the Cap structure or poly (A), the amount of enzyme used should also be analyzed.
The sponsor may be encouraged to develop state-of-the-art technology methods, if necessary, and conduct quality assessment and quality control using these methods. The development of assay methods may include the preparation of new reagents and other materials to analyze process-derived impurities.
As mRNA is easily degraded by RNase and is not efficiently internalized into cells, it is encapsulated into LNPs during the formulation process to ensure in vivo stability, delivery to target cells, and sustained intracellular release. In the encapsulation of purified mRNA DS into LNPs, multiple lipid molecules are used to anticipate various changes in the behavior of mRNA in the human body, and a formulation using microfluidic channels with baffle structures is used to efficiently encapsulate mRNA into lipid molecules (Fig. 3).

Outline of the production of an messenger ribonucleic acid (mRNA)-LNP (lipid nanoparticle) formulation using microfluidic channels.
When seeking the approval of a vaccine, vaccine developers must be prepared to (i) explain the chemical characteristics of each lipid applied to the design of the formulation process using LNPs, (ii) clarify whether the desired properties have been obtained in terms of the physical parameters of the mRNA-encapsulated nanoparticles, and (iii) demonstrate the purpose of the formulation’s design and the justifications for its specifications. Therefore, it is necessary to confirm the consistency and stability of the formulation quality of the final product. In addition to evaluating the potential toxicity of the lipids that comprise the LNPs, it may be useful to characterize the function of each lipid component with positive and/or negative effects on the immune response and vaccine efficacy.
The role and safety of the lipid components used in vaccine formulations must be evaluated. For example, if one of the lipid components has an adjuvant effect, the lipid content of the LNP may be an important quality characteristic. Thus, the consistency of the lipid composition and content in the formulated particles should also be evaluated.
Because DNA templates used for in vitro transcription reactions are important raw materials for the DS of mRNA, it is important to evaluate whether the template DNA is sufficiently removed during the purification process from the standpoint of quality and safety. To ensure the quality of the DS, it is reasonable to set acceptance criteria for the templates. Product- and process-related impurities such as incomplete sequences and endotoxins are examples of items that should be addressed in DS specifications.
The specifications for a DS are expected to include the identity of the DS, its content, physical and chemical properties, biological activity, and product- and process-related impurities. In particular, it is necessary to consider (i) whether the addition of a 5′-Cap or poly (A) is appropriate, (ii) the mRNA purity (percentage of intact mRNA), and (iii) other impurities (e.g., the percentage of dsRNA, RNA shorter or longer than the desired length, and template DNA) (Table 1). However, if some quality attributes can be adequately controlled using in-process control tests, it would be acceptable not to conduct a release test. Vaccine developers must establish specifications that guarantee sufficient safety margins for impurities. It is also necessary to determine whether analytical procedures have sufficient sensitivity and accuracy.
| WHO | Comirnaty® intramuscular injection | Moderna Spikevax intramuscular injection |
|---|---|---|
| Drug substance: | ||
| Identity | Identity | Identity |
| Purity and impurities | RNA integrity, purity (double- strand RNA and template DNA), 5′-Cap and poly (A) tail | Purity (mRNA purity, product-related impurities, residual DNA, 5′-Cap rate additional rate, poly (A) tail) |
| Quantification and physical state | Contents | Contents |
| Safety attributes | Bioburden, endotoxins | Bioburden, endotoxins |
| Additional quality attributes | Clarity, pH | Characteristics, pH |
| Drug product: | ||
| Identity | Identity (RNA) | Identity (RNA) |
| Purity and impurities | RNA integrity | Purity test (RNA, product-related impurities) |
| Quantification and physical state | Content | Content |
| Safety attributes | Sterility test and endotoxin test | Sterility test and endotoxin test |
| Additional quality attributes | Appearance, pH, identity (lipid), lipid content, particle size and polydispersity, RNA encapsulation, osmolality, insoluble particle matter, vial content volume | Characteristics, pH, identity (lipid), lipid content, purity test (lipid impurity), particle size and polydispersity, encapsulated RNA, osmolality, insoluble foreign matter, insoluble particulate matter, vial content volume |
| Potency | Biological activity | In vitro translation |
This table was compiled from Ref. [35] and the review report for Comirnaty® intramuscular injection and the Moderna COVID-19 vaccine intramuscular injection with some modifications. Masked information in the review report is not included in this table. WHO: World Health Organization; RNA: ribonucleic acid; DNA: deoxyribonucleic acid.
Although tests for the drug products listed in Table 1 are envisioned, the table does not cover all the tests, and not all tests are necessary. However, tests for identifying the contents of a drug product are essential for dose setting and may be useful for demonstrating the accuracy and validity of the drug product. It is important to consider how formulation processes affect the biological activity and constancy of a drug product.
Reference materialsReference materials are often prepared using highly purified mRNA DSs that have been fully characterized from orthogonal points. For example, the formulation batches used in clinical trials can be fully characterized by their chemical composition, purity, biological activity, complete sequence analysis, and other parameters for use as reference materials regarding their physical and/or biological properties.
Stability testing and validity periodsSince mRNA DSs and products have characteristics that are sensitive to storage temperatures, a shelf-life that can basically guarantee that a certain level of quality is maintained should be set; it should be considered to ensure the consistency of efficacy and safety based on a thorough understanding of the stability profile from the results of long-term storage studies evaluated under actual storage conditions and periods, as is the case with biopharmaceuticals. Conducting stability studies at multiple temperatures based on actual usage scenarios and discussing the results of studies conducted under freeze-thaw conditions may provide useful information for understanding the stability of drug substances and products and the control of the appropriate storage temperatures of drug products in clinical settings.
In Japan, non-clinical studies on mRNA vaccines are conducted in accordance with the country’s Guidelines for Non-clinical Studies of Vaccines for the Prevention of Infectious Diseases [32]. They provide point-to-consider principles for evaluating vaccines against COVID-19 [33]. Nevertheless, the safety and efficacy of mRNA vaccines should be evaluated by considering their unique quality characteristics. For example, in the case of LNP-mRNA vaccines, if the LNPs include chemical substances that have not been previously used in humans, a non-clinical safety evaluation of these chemical substances, which is required for a new additive in a drug product, would be necessary. In addition, if chemical modifications are used for the mRNA (including 5′-Cap and poly (A)), a safety evaluation focusing on the modifications should be considered.
In mRNA vaccines approved in Japan (as of May 2023), uridine in the mRNA is replaced with pseudouridine to reduce the high innate immune activation potential of the mRNA, as mentioned above. Because pseudouridine is a naturally occurring nucleic acid, there are no genotoxicity concerns. There is concern that innate immune activation by mRNA may cause adverse effects such as inflammatory reactions, including fever and pain, at the administration site. However, there are limitations in extrapolating test results from animals to humans; thus, careful evaluation of these reactions in clinical trials is necessary.
In the development of a SARS-CoV-2 vaccine during a pandemic period, if the platform technology used to manufacture the vaccine is already approved or its use in a clinical trial is sufficiently well defined, non-clinical safety study results of other products manufactured using the same platform technology can be used as data to determine whether to initiate clinical trials [3]. The WHO has stated that for mRNA vaccines other than the SARS-CoV-2 vaccine, by utilizing the same platform concept, non-clinical safety studies can be omitted if agreed upon by the national regulatory authority [34].
Clinical trials on mRNA products are required in accordance with Japan’s Guidelines for Clinical Trials of Vaccines for the Prevention of Infectious Diseases [35]. However, as in non-clinical studies, if matters are to be considered specific to mRNA vaccine products, they should be evaluated separately.
This study was supported by a grant from the Japan Agency for Medical Research and Development (AMED), No. 22mk010194j002).
None.
This paper is described as a review, with further discussion based on a Japanese article (PMDRS 2023:54 (4): 312–321). Therefore, we thank Junichi Fukuchi, Takaaki Yoshida, Mihiro Kawamura, and Kengo Kawachi (Office of Vaccines and Blood Products, Japan Pharmaceuticals and Medical Devices Agency) for their assistance in writing this manuscript.