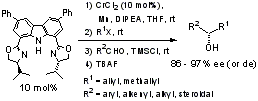
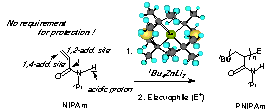29th Symposium on Progress in Organic Reactions and Syntheses
Displaying 101-115 of 115 articles from this issue
Poster Presentations
-
Pages 204-205
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 206-207
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 208-209
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 210-211
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 212-213
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
Oral Presentations
-
Pages 214-215
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 216-217
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 218-219
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 220-221
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 222-223
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 224-225
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 226-227
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 228-229
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 230-231
Published: September 24, 2003
Released on J-STAGE: March 16, 2004
-
Pages 232-233
Published: September 24, 2003
Released on J-STAGE: March 16, 2004