
- Issue 6 Pages 112C1-
- Issue 5 Pages 181-
- Issue 4 Pages 139-
- Issue 3 Pages 109-
- Issue 2 Pages 59-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
XAFS study on the location of Cu and Mn in a greenish blue elbaite from Alto dos Quntos mine, BrazilKazumasa SUGIYAMA, Hiroshi ARIMA, Hayato KONNO, Takashi MIKOUCHI2017Volume 112Issue 4 Pages 139-146
Published: 2017
Released on J-STAGE: September 02, 2017
JOURNAL FREE ACCESSThe local structure of Cu and Mn in a greenish–blue elbaite from Alto dos Quintos, Brazil, was studied by using X–ray absorption fine structure (XAFS) spectroscopy. The observed local structural features suggest the distribution of Cu2+, Mn2+, and Mn3+ at the distorted Y–site.
View full abstractDownload PDF (3611K) -
Atsuyuki INOUE, Minoru UTADA2017Volume 112Issue 4 Pages 147-158
Published: 2017
Released on J-STAGE: September 02, 2017
Advance online publication: July 07, 2017JOURNAL FREE ACCESS
Supplementary materialOccurrence and mineralogical properties of pinkish colored epidotes that were found in core samples from a geothermal exploration well NB–1, Noboribetsu, Hokkaido, Japan are described together with estimating the formation conditions. The minerals occurred mainly as vein– and druse–fillings in volcaniclastic rocks of the Miocene Osarugawa Formation. They coexisted with brownish chlorites, K–feldspar, illite, and quartz, and associated with minor hematite, titanite, and/or apatite. The minerals were produced by local K–alteration that overprinted early–formed regional propylitization associated with ordinary greenish epidotes and chlorites. The cell parameters of pinkish epidote were: a = 8.884(6), b = 5.614(1), c = 10.149(7) Å, β = 115.5(8)°, and V = 456.9(4) Å3. The Ca2Mn3+3Si3O12(OH) contents of the pinkish epidotes were generally less than 2.4 mol%, while the Ca2Fe3+3Si3O12(OH) contents attained 28 mol%. It is considered that the low Mn contents and relatively low Fe contents resulted in giving the characteristic pinkish to pale yellow colors for epidote. Associated brownish chlorites were characterized by low total Fe and relatively high Fe3+ contents. Their formation temperatures were estimated to be 230–300 °C, using semi–empirical chlorite geothermometers. These data indicate that pinkish epidotes coexisted with brownish chlorites formed under oxidative conditions and probably low Fe contents in hydrothermal fluids.
View full abstractDownload PDF (5472K) -
Ayaka HAYASHI, Koichi MOMMA, Ritsuro MIYAWAKI, Mitsuo TANABE, Shigetom ...2017Volume 112Issue 4 Pages 159-165
Published: 2017
Released on J-STAGE: September 02, 2017
Advance online publication: August 19, 2017JOURNAL FREE ACCESSKurchatovite occurs as colorless granular crystals up to 1 mm at the Fuka mine, Okayama Prefecture, Japan. The mineral is associated with shimazakiite, calcite and johnbaumite. The Vickers microhardness is 441 kg mm−2 (50 g load), corresponding to 4½ on the Mohs’ scale. The calculated density is 3.23 g cm−3. Electron microprobe analyses of kurchatovite gave empirical formulae ranging from Ca0.987(Mg1.004Fe0.020Mn0.001)Σ1.025B1.992O5 to Ca0.992(Mg0.466Fe0.523)Σ0.989B2.013O5 based on O = 5, and kurchatovite forms a continuous solid solution in this range. The mineral is orthorhombic, Pbca, and the unit cell parameters refined from XRD data measured by a Gandolfi camera are a = 36.33(14), b = 11.204(3), c = 5.502(17) Å, V = 2239(12) Å3. It is formed by a layer of MgO6–octahedra, a layer of CaO7–polyhedral and B2O5 consisting of two BO3–triangles. The kurchatovite from the Fuka mine was probably formed by supplying Mg and Fe to shimazakiite from hydrothermal solution at a temperature between 250 to 400 °C.
View full abstractDownload PDF (1428K) -
Shunsuke ENDO2017Volume 112Issue 4 Pages 166-174
Published: 2017
Released on J-STAGE: September 02, 2017
Advance online publication: August 10, 2017JOURNAL FREE ACCESSIlvaite, a hydrous calcium mixed–valent iron silicate, is found in red hematitic chert (jasper) lenses in altered basalt and an iron–manganese ore from the Sumaizuku unit (Middle Jurassic accretionary complex) of the Northern Chichibu belt, SW Japan. The jasper lenses are composed of ilvaite, andradite, stilpnomelane, riebeckite, hematite and quartz. Ilvaite in the jasper lenses shows an unusual spherical morphology (~ 100 µm in diameter) and each ilvaite spherule exhibits a foam–like microstructure with recrystallized quartz grains. Ilvaite also occurs as discrete euhedral crystals (up to 500 µm in length) in quartz segregation veins or monomineralic ilvaite veins. Ilvaite in the jasper lenses is close to the endmember composition, but the material in the iron–manganese ore is rich in [6]Mn2+ (up to 0.84 atoms per formula unit) corresponding to manganilvaite. Thermodynamic modeling shows that the ilvaite–andradite–hematite–quartz association (ilvaite + O2 = hematite + andradite + quartz) is stable under the peak P–T conditions of the Sumaizuku unit (~ 0.35 GPa, 230–250 °C) and oxygen fugacity slightly above the hematite–magnetite buffer (log fO2 = ~ –38). Ilvaite spherules are intimately associated with colloform–textured andradite, suggesting that they probably originated from a Ca–Fe–Si colloidal precursor deposited along hydrothermal fluid conduits within the Early Permian oceanic crust, and recrystallized during subduction–related low–grade metamorphism.
View full abstractDownload PDF (5682K) -
Takeshi IKEDA, Kazuhiro MIYAZAKI, Hirohisa MATSUURA2017Volume 112Issue 4 Pages 175-179
Published: 2017
Released on J-STAGE: September 02, 2017
JOURNAL FREE ACCESSThis study evaluates uncertainty in pressure difference of empirical geobarometers derived from thermodynamic properties, which is expressed as:
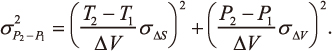
The uncertainty is independent of enthalpy and its uncertainty, and increases mainly with increasing temperature difference. Examination of several empirical geobaromters reveals that the uncertainty in pressure difference is smaller than that in pressure estimation by a factor of at least one third within the temperature range of almost every single metamorphic complex.
View full abstractDownload PDF (2997K)
- |<
- <
- 1
- >
- >|