2022 Volume 55 Issue 5 Pages 129-148
2022 Volume 55 Issue 5 Pages 129-148
The enzyme-labeled antigen method is an immunohistochemical technique detecting plasma cells producing specific antibodies in tissue sections. The probe is an antigen labeled with an enzyme or biotin. This immunohistochemical technique is appliable to frozen sections of paraformaldehyde (PFA)-fixed tissues, but it has been difficult to apply it to formalin-fixed, paraffin-embedded (FFPE) sections. In the current study, factors inactivating the antibody reactivity during the process of preparing FFPE sections were investigated. Lymph nodes of rats immunized with horseradish peroxidase (HRP) or a mixture of keyhole limpet hemocyanin/ovalbumin/bovine serum albumin were employed as experimental models. Plasma cells producing specific antibodies, visualized with HRP (as an antigen with enzymatic activity) or biotinylated proteins in 4% PFA-fixed frozen sections, significantly decreased in unbuffered 10% formalin-fixed frozen sections. The positive cells were further decreased by paraffin embedding following formalin fixation. In paraffin-embedded sections fixed in precipitating fixatives such as ethanol and acetone and those prepared with the AMeX method, the antigen-binding reactivity of antibodies was preserved. Fixation in periodate-lysine-paraformaldehyde and Zamboni solution also kept the antigen-binding reactivity in paraffin to some extent. In conclusion, formalin fixation and paraffin embedding were major causes inactivating antibodies. Precipitating fixatives could retain the antigen-binding reactivity of antibodies in paraffin-embedded sections.
Antibody-producing plasma cells are clustered in chronic inflammatory lesions of infectious diseases, autoimmune diseases, and malignant tumors [4, 30–32]. Antigens recognized by the antibodies produced in the immunocytes remain unknown in most instances. Sites of production of disease-specific antibodies, such as antibodies against pathogenic bacteria in bacterial infections and autoantibodies in autoimmune diseases, remain unsettled either. Simply because these immunocytes are located within the lesions, it is highly likely that they secrete disease-specific antibodies. Distributions of plasma cells producing specific antibodies can be visualized by using the enzyme-labeled antigen method, which is an immunohistochemical technique to detect plasma cells producing specific antibodies in tissue sections using antigenic molecules labeled with biotin [7]. It represents a reversed technique of enzyme-labeled antibody method called as “immunohistochemical staining” commonly applied to experimental and diagnostic pathology and related research [6].
Using the enzyme-labeled antigen method, we have demonstrated plasma cells producing disease-specific antibodies in frozen sections of diseased tissues. In gingival tissues of radicular cyst and periodontitis, plasma cells producing antibodies reactive with antigens of Porphyromonas gingivalis, a Gram-negative anaerobic bacterium causing periodontitis, were identified [9, 26]. In the inflamed synovium of rheumatoid arthritis, autoantibody-producing plasma cells were visualized [8]. In Streptococcus pyogenes-induced tonsilitis, plasma cells producing antibodies against strep A, a bacterial carbohydrate antigen, were localized [12]. Plasma cells producing disease-specific antibodies were visualized in frozen sections of 4% paraformaldehyde (PFA)-fixed tissue specimens but not in formalin-fixed paraffin-embedded (FFPE) sections. At present, it is difficult for us to apply the enzyme-labeled antigen method to FFPE sections. Supposedly, antigen-binding reactivity of the antibodies in tissues is deteriorated during the process of preparing FFPE sections. Under the experimental conditions, however, the plasma cells producing antibodies against specific antigens were partly detectable in FFPE sections [7]. There are plentiful reports stating the effect of fixatives on the antigenicity of protein molecules in tissue sections and antigen retrieval methods in FFPE sections for applying the enzyme-labeled antibody method [13, 14, 22, 29]. However, it has not been discussed enough for the effect of fixatives on the antigen-binding reactivity of antibodies in tissue sections. In the present study, various factors affecting the antigen-binding reactivity of antibodies in FFPE sections were evaluated.
FFPE blocks of tissues with various disorders, including those of rare diseases, have been kept for a long period of time in the diagnostic pathology divisions in hospitals. Once the enzyme-labeled antigen method is applicable to FFPE sections, we can analyze those specimens retrospectively. Application of the enzyme-labeled antigen method to FFPE sections is thus keenly anticipated.
The enzyme-labeled antigen method is applicable to frozen sections, but as mentioned above, we have certain limitations. The frozen tissue blocks are requested to be stored at −80°C for maintaining the quality of samples [18]. For cryostat sectioning, frozen tissue blocks are moved from a deep freezer at −80°C to a cryostat at −20°C. Such a change of the temperature may hamper the quality of samples. In contrast, paraffin-embedded tissue samples can be stably kept at room temperature. It is thus important for us to determine the fixation condition for preparing paraffin-embedded sections suitable for the enzyme-labeled antigen method.
In the current study, we investigated factors deteriorating the antigen-binding reactivity of antibodies in tissues during the process preparing FFPE sections. Samples used herein were lymph nodes of rats experimentally immunized with specific antigens. To pursue adequate fixation conditions, tissues were fixed in a variety of fixatives to prepare paraffin-embedded sections. Effects of en bloc heating on the retrieval of the antigen-binding reactivity in formalin-fixed samples and FFPE tissues, as well as those of the pretreatment by periodic acid oxidation, enzymatic proteolysis and hydrated heating of paraffin sections, were evaluated. In order to detect signals with high sensitivity, three kinds of signal amplification methods, such as the amino acid polymer method, avidin biotinylated peroxidase complex (ABC) method and catalyzed signal amplification method-II (CSA-II), were attempted.
Unbuffered 10% formalin and neutral-buffered 10% formalin were prepared by diluting 35–38% formaldehyde solution (Nacalai Tesque, Kyoto, Japan) with deionized water and 0.01 M phosphate buffer, pH 7.2, respectively. Phosphate-buffered 4% PFA was prepared by dissolving paraformaldehyde (Merck, Darmstadt, Germany) in 0.1 M phosphate buffer, pH 7.4. Four percent carbodiimide solution was prepared by dissolving carbodiimide (Tokyo Chemical Industry, Tokyo, Japan) in deionized water. Zamboni solution containing picric acid and paraformaldehyde at neutral pH, and 2% paraformaldehyde-lysine-periodate (PLP) solution were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Carnoy’s solution was prepared by admixing 60% ethanol (Nacalai Tesque), 30% chloroform (Nacalai Tesque) and 10% glacial acetic acid (Nacalai Tesque). Ethanol and acetone were purchased from Nacalai Tesque and Katayama Chemical Industries (Osaka, Japan), respectively. Acetone-based modified fixation, acetone, methyl benzoate and xylene (AMeX) method [16], was also employed. Lymph nodes were soaked in acetone at 4°C for 20 min and then at −20°C overnight, followed by soaking in acetone at 4°C for 15 min and at room temperature for 15 min. Tissues were then cleared in methyl benzoate at room temperature for 15 min twice and then in xylene at room temperature for 15 min twice. Tissues were finally embedded in paraffin. PLP-AMeX method was further performed, as reported previously [23]. Tissues were fixed in PLP solution at 4°C for 4 hr, then rinsed in cold 0.01 M phosphate-buffered saline (PBS), pH 7.2, for 30 min. Tissues were then soaked in cold acetone, cleared in methyl benzoate and xylene, and finally embedded in paraffin.
Antigens and probesHorseradish peroxidase (HRP) were purchased from Fujifilm Wako Pure Chemical Corporation. Keyhole limpet hemocyanin (KLH), ovalbumin (OA) and bovine serum albumin (BSA) were purchased from Merck. HRP and a mixture of KLH, OA and BSA were subcutaneously immunized in rats, as described below. HRP was directly used as a probe for the labeled antigen method, because it is an enzyme by itself. KLH, OA and BSA were labeled with biotin by EZ-Link Sulfo-NHS-LC-Biotinylation Kit (Thermo Fisher Scientific, Tokyo, Japan), according to the manufacturer’s instruction, and used as biotinylated probes for the labeled antigen method.
Experimental animalsMale Sprague-Dawley rats (Chubu Kagaku Shizai, Nagoya, Japan), 5 weeks old, were kept in the animal laboratory of Fujita Health University, Toyoake, Japan. The animal experiments were conducted in accordance with the Guidelines for the Management of Laboratory Animals in Fujita Health University (Approval No. AP16060).
ImmunizationImmunization of specific antigens was performed, as reported previously [7]. A total of 18 or 12 rats were immunized with HRP or the mixture of KLH, OA and BSA, respectively. One mg HRP or mixture of 1 mg each of KLH, OA and BSA were dissolved in 1 ml saline and emulsified with an equal quantity of Freund’s complete adjuvant (Becton, Dickinson and Company, NJ, USA). The emulsions (0.1 ml) were injected three times into the footpad of all four legs of rats. At the second and third injections, 1 and 5 weeks after the initial injection, Freund’s complete adjuvant was replaced by Freund’s incomplete adjuvant (Becton, Dickinson and Company).
Tissue sampling and preparation of 4% PFA-fixed tissue sectionsTwo weeks after the third antigen inoculation, the rats were euthanized by inhalation with isoflurane (Pfizer, Tokyo, Japan). Bilateral popliteal, groin, and axillary lymph nodes were sampled and rinsed in PBS. Each lymph node was sliced into two pieces and one of them was soaked into 4% PFA at 4°C for 4 hr. The other part was used in the experiments described below for evaluating factors deteriorating the antigen-binding reactivity in tissue sections. The PFA-fixed tissues were soaked in PBS containing 10% sucrose at 4°C overnight and additionally soaked in PBS containing 15% and 20% for 4 hr each. The rinsed tissues were embedded in an embedding medium, Tissue Mount (Chiba Medical, Saitama, Japan), quickly frozen with dry ice-acetone, sectioned on a cryostat (Leica Microsystems, Wetzlar, Germany) at 3 μm thickness, and mounted on 3-aminopropyl-triethoxysilane–coated glass slides (Muto Pure Chemicals). The frozen sections were dried for 30 min with a drier at room temperature and stored at −20°C until staining. The PFA-fixed frozen sections functioned as a benchmark for the reactivity of antigen-specific antibodies, in order to compare with the reactivity in sections of tissues treated under various conditions.
Enzyme-labeled antigen methodThe enzyme-labeled antigen method using frozen sections was performed, as reported previously [10]. After rinsing in running water, endogenous peroxidase activity in sections was quenched in methanol containing 0.3% H2O2 at room temperature for 30 min. The sections were then treated with 0.05 M Tris-buffered saline (TBS), pH 7.6, containing 1 μg/ml proteinase K at room temperature for 15 min, when needed. After rinsing in PBS, the sections were incubated with PBS containing 1 μg/ml HRP, 50 μg/ml biotinylated KLH, 50 μg/ml biotinylated OA, or 10 μg/ml biotinylated BSA at room temperature for 1 hr, and rinsed in PBS three times for 15 min each. To detect the biotinylated antigens (KLH, OA and BSA), sections were reacted with undiluted HRP-labeled streptavidin (Nichirei Bioscience, Tokyo, Japan) at room temperature for 1 hr. After rinsing in PBS, the sections were incubated in 0.05 M Tris-HCl buffer, pH 7.6, containing 0.02% diaminobenzidine (DAB) and 0.006% H2O2. The nuclei were briefly counterstained with Mayer’s hematoxylin. Sections were dehydrated through graded ethanol, penetrated with xylene, and mounted in hydrophobic mounting medium, Entellan New (Merck).
Paraffin sections were deparaffinized in xylene and soaked in ethanol, and thereafter the same protocol as for the frozen sections was performed.
Detection of antibody-producing cellsAfter a brief dip in running water and endogenous peroxidase quenching, the frozen sections were treated with TBS containing 1 to 5 μg/ml proteinase K at room temperature for 15 min, when needed. After rinsing in PBS, the sections were incubated overnight with 1:2,000 to 1:4,000-diluted biotinylated rabbit monoclonal antibodies against rat kappa and lambda light chains (a cocktail of clones RT39 and RL-6, Merck), in order to know the total number of plasma cells. The sections were then rinsed in PBS three times and incubated with HRP-labeled streptavidin. Chromogenic DAB reaction and brief hematoxylin counterstaining followed.
Paraffin sections were deparaffinized in xylene and penetrated in ethanol, and then proteinase K treatment (5 μg/ml) was performed at room temperature for 15 min, when needed. After rinsing in PBS, the sections were incubated with 1:500 diluted monoclonal antibodies against rat kappa and lambda light chains at room temperature overnight.
Evaluation of factors deteriorating the antigen-binding reactivity of antibodies during the process of preparing FFPE sectionsFive lymph nodes were sampled from HRP-immunized rats (n = 3). Each lymph node was divided into two pieces. One piece was processed into 4% PFA-prefixed frozen sections, as described above. Another was soaked in unbuffered 10% formalin at room temperature for 24 hr and briefly rinsed in tap water. One of the unbuffered 10% formalin-fixed tissue pieces was processed for frozen sections to evaluate the effect of the fixation in unbuffered 10% formalin on the antibody reactivity. For evaluating the effect of heating during paraffin embedding on the antibody reactivity, another piece was incubated en bloc in deionized water, instead of paraffin, at 60°C for 4 hr, and was then processed to preparing frozen sections. The other three unbuffered formalin-fixed pieces were dehydrated through 100% ethanol seven times for an hour each. For evaluating the effect of the ethanol treatment, one of the dehydrated tissues was processed to preparing frozen sections. The remaining two were soaked in xylene three times for 1.5 hr each. For evaluating the effect of the xylene treatment, one of them was re-soaked in ethanol seven times for an hour each, and was then processed to preparing frozen sections. The last piece was processed to preparing FFPE sections. The tissue sections were reacted with HRP, as well as with diluted monoclonal antibodies against both rat kappa and lambda light chains, and the stainability of plasma cells producing anti-HRP antibodies was evaluated. The lymph nodes of three HRP-immunized rats were comparatively evaluated for the respective test conditions. The detectability of anti-HRP antibody-producing plasma cells was calculated and compared among the respective test conditions. The procedures of the tissue processing are illustrated in Figure 1.

Schematic presentation of the procedures of tissue processing for the evaluation of factors deteriorating the antigen-binding reactivity of antibodies in the process of preparing FFPE sections. Five lymph nodes were sampled from the HRP-immunized rats. Each lymph node was divided into two pieces. One of them was processed to preparing 4% PFA-prefixed frozen sections. The other piece was soaked in unbuffered 10% formalin at room temperature for 24 hr. One of the unbuffered 10% formalin-fixed tissue pieces was processed for frozen sections (a). Another piece was incubated in deionized water at 60°C and was then processed to preparing frozen sections (b). The other three formalin-fixed pieces were dehydrated through 100% ethanol. One of dehydrated tissues was processed to preparing frozen sections (c). The remaining two were soaked in xylene. One of them was rehydrated in ethanol and was then processed to preparing frozen sections (d). The last piece was processed to preparing FFPE sections (e).
Four lymph nodes were sampled from the HRP-immunized rat (n = 3). Each lymph node was divided into two pieces and one of them was processed to preparing 4% PFA-fixed frozen sections. The other was soaked in unbuffered 10% formalin for 4, 24 and 48 hr and one week at room temperature. The unbuffered formalin-fixed tissues were rinsed in PBS containing 10%, 15% and 20% sucrose, and frozen sections were prepared. These sections were stained with HRP and antibodies against kappa and lambda light chains. The detectability of anti-HRP antibody-producing plasma cells was calculated and compared among the test conditions.
Evaluation of the effect of formaldehyde-based fixatives on the antibody reactivitySix lymph nodes were sampled from the HRP immunized rat (n = 3). Each lymph node was divided into two pieces, and one of them was processed to preparing 4% PFA-fixed frozen sections. Another piece was fixed in 4% PFA at 4°C for 24 hr, and buffered or unbuffered 10% formalin at room temperature for 24 hr. The fixed tissues were processed to preparing frozen and paraffin-embedded sections. The frozen and paraffin-embedded sections were stained with HRP and antibodies against kappa and lambda light chains. Anti-HRP antibody-producing plasma cells in frozen and paraffin sections were calculated and compared among the test conditions.
Evaluation of the effect of other fixatives on the antibody reactivityTwo to six lymph nodes were sampled from the rat (n = 3) immunized with HRP or the mixture of KLH, OA and BSA. Each lymph node was sliced into two pieces, and one of them was processed to preparing 4% PFA-fixed frozen sections. The other part was soaked in PLP solution, Zamboni solution, 4% carbodiimide solution, Carnoy’s solution, ethanol or acetone, and was also processed for the AMeX or PLP-AMeX methods. Part of the methyl benzoate-treated tissues in the AMeX and PLP-AMeX methods were rinsed in sucrose-PBS to be processed for frozen sections.
Frozen and paraffin-embedded sections were prepared from the respective specimens, and the enzyme-labeled antigen method was performed using the corresponding antigens. The antibody reactivity in each section was evaluated in comparison with that in 4% PFA-fixed frozen sections of the corresponding lymph node.
The lymph node from immunized rats (n = 3) was evaluated under the respective test conditions. Procedures and conditions of treatments in the respective fixatives are summarized in Supplementary Table S1.
Evaluation of en bloc heating of formalin-fixed tissues for retrieving the antigen-binding reactivity of antibodiesThree lymph nodes were evaluated for the respective test conditions. Lymph nodes sampled from rats immunized with HRP or KLH/OA/BSA were soaked in unbuffered 10% formalin at room temperature for 24 hr. After a brief rinse in tap water, the tissues were heated in PBS at 70, 80 or 90°C for 4 hr. After heating, frozen and paraffin-embedded sections were prepared, and the sections were reacted with the corresponding antigen probes. The antibody reactivity in each section was evaluated in comparison with the antibody reactivity in 4% PFA-fixed frozen sections of the corresponding lymph node.
Preparing frozen sections from FFPE tissue blocksThree lymph nodes were used for the respective test conditions (n = 3). FFPE lymph nodes of rats immunized with HRP or KLH/OA/BSA were processed to preparing frozen tissue blocks in the following. FFPE lymph nodes were soaked in xylene to dissolve paraffin, and then rehydrated with ethanol. The rehydrated tissues were kept at room temperature, or heated in PBS at 70°C, 80°C or 90°C for 4 hr. After the en bloc heating treatment, the tissues were rinsed in PBS containing 10, 15 and 20% sucrose and quickly frozen in dry-ice acetone to prepare frozen sections. The sections were reacted with the labeled antigens: HRP, biotinylated KLH, biotinylated OA or biotinylated BSA. The antibody reactivity in each section was evaluated, in comparison with the antibody reactivity in 4% PFA-fixed frozen sections of the corresponding lymph node.
Changes of the antigen-binding reactivity of antibody in tissue sections after proteinase K treatment and changes of the signal intensity by probe concentrationsWe evaluated the effect of proteinase K digestion on the antibody reactivity in paraffin-embedded sections of the lymph node of HRP immunized rats (n = 3) after fixation in 4% PFA and buffered or unbuffered 10% formalin. The enzyme-labeled antigen method was performed with varied concentrations of proteinase K and the probe (HRP). Namely, the tissue sections were treated with 1 to 320 μg/ml proteinase K at room temperature for 15 min. After pretreating with 80 μg/ml proteinase K, sections were incubated with HRP at the concentration of 1, 10 and 100 μg/ml.
Changes of the antigen-binding reactivity of antibodies in FFPE sections after hydrated heatingPressure cooker-mediated hydrated heating was evaluated whether or not the antigen-binding reactivity of antibodies in FFPE sections was retrieved. Two kinds of soaking solution were checked, including 10 mM citrate buffer, pH 6.0 and 1 mM ethylenediamine tetraacetic acid (EDTA) solution, pH 8.0. The pressure pan was provided from T-Fal company (Rumilly, Haute-Savoie, France). The deparaffinized sections were heated under pressure at 121°C for 10 min.
Changes of the antigen-binding reactivity of antibodies in FFPE sections after periodic acid oxidationWe evaluated whether the oxidation by periodic acid might affect the antibody reactivity in FFPE sections of the lymph node of HRP-immunized rats. Sections were treated with 0.5% periodic acid (FUJIFILM Wako Pure Chemical Corporation) at room temperature for 10 min prior to incubation with HRP. The combination of the periodate treatment and heating or 20 μg/ml proteinase K treatment was also evaluated: 10 mM citrate buffer, pH 6.0 and 1 mM EDTA, pH 8.0 were used as a heating buffer solution.
Application of three kinds of signal amplification methods to detecting antigen-specific antibody-producing cells in unbuffered 10% formalin-fixed paraffin-embedded tissue sectionsIn order to evaluate the increased sensitivity for detecting antigen-specific antibody-producing cells, three kinds of signal amplification techniques were applied. These included the polymer-based method (amino acid polymer method), avidin-biotinylated peroxidase complex (ABC) method and catalyzed signal amplification (CSA)-II method [1, 3, 15]. Tissue sections of the unbuffered 10% formalin-fixed lymph node from rats immunized with the mixture of KLH, OA and BSA were evaluated. Deparaffinized sections after endogenous peroxidase quenching were treated with TBS containing 20 μg/ml proteinase K at room temperature for 15 min. After rinsing in PBS three times, the sections were incubated with 50 μg/ml biotinylated KLH, 50 μg/ml biotinylated OA or 10 μg/ml biotinylated BSA. After a brief rinse in PBS, the sections were incubated at room temperature for 30 min with 1:2,000 diluted polyclonal rabbit anti-biotin antibody (Abcam, Cambridge, UK). Three kinds of the signal amplification techniques followed.
For applying the amino acid polymer method, the sections were incubated with the amino acid polymer conjugated with HRP and the Fab region of anti-rabbit IgG (Histofine, Simple Stain Rat MAX-PO (R), Nichirei Bioscience, Tokyo) at room temperature for one hour. After rinsing in PBS three times, chromogenic DAB reaction and hematoxylin counterstaining followed.
For applying the ABC method, the sections were incubated with 1:500-diluted biotinylated anti-rabbit IgG (Vector laboratories, CA, USA) at room temperature for 30 min. Thereafter, the sections were incubated with avidin-biotinylated peroxidase complex (VECTASTAIN® Elite ABC-HRP Kit, Peroxidase (Standard), Vector Laboratories) at room temperature for 30 min. Chromogenic DAB reaction and hematoxylin counterstaining followed.
For applying the CSA-II method, the sections were incubated with 1:1,600 diluted, HRP-conjugated mouse monoclonal anti-rabbit IgG (Merck) at room temperature for 30 min. The sections were rinsed in TBS and then in TBS containing 0.1% Tween 20 twice. The sections were incubated with fluorescein isothiocyanate (FITC)-conjugated tyramide (Amplification reagent, CSA-II, biotin-free Tyramide Signal Amplification System, Agilent, CA, USA) at room temperature for 15 min. After rinsing in TBS and then in TBS containing 0.1% Tween 20, the sections were incubated with HRP-conjugated anti-FITC antibody at room temperature for 15 min. The sections were rinsed in TBS and then in TBS containing 0.1% Tween 20. Finally, the chromogenic DAB reaction and hematoxylin counterstaining followed.
The procedures are summarized in Supplementary Table S2.
Proportion of plasma cells producing anti-HRP antibodies in total antibody-producing plasma cellsAs the proportion of anti-HRP antibody-producing plasma cells in total antibody-producing plasma cells varied among the lymph nodes, it seemed inadequate that the proportions were directly used as the detectability of plasma cells producing anti-HRP antibodies.
Therefore, the detectability of anti-HRP antibody-producing plasma cells in sections of the lymph node was calculated in the followings. As shown in Figure 1, the respective lymph nodes were divided into two pieces. One was processed into 4% PFA-fixed frozen sections as a benchmark of the detectability of anti-HRP antibody-producing cells in the respective lymph node, and the other part was treated under various conditions. Consecutive frozen or paraffin sections of the respective lymph node were stained with HRP and cocktail monoclonal antibodies against kappa and lambda light chains to detect anti-HRP antibody-producing plasma cells and total antibody-producing cells, respectively. Positive cells were counted in the respective sections under a high-powered (× 400) microscopic field. The same areas were chosen for evaluation. The proportion of anti-HRP antibody-producing plasma cells in total antibody-producing cells was thus evaluated. The proportion of anti-HRP antibody producing cells in total antibody producing plasma cells was adjusted by comparing with the corresponding 4% PFA-fixed frozen sections to express as a percentage. The percentage value was used as the detectability of anti-HRP antibody-producing cells under the respective conditions.
Namely, the following formula was employed to calculate the detectability of anti-HRP antibody producing cells under the respective conditions. The detectability of anti-HRP antibody producing cells = the proportion of anti-HRP antibody producing cells in paraffin sections/the proportion of anti-HRP antibody-producing cells in the corresponding 4% PFA-fixed frozen sections × 100.
Evaluation of the detectability of plasma cells producing antibodies against specific antigensSuch treatments as fixation in Carnoy’s solution and high temperature treatment abolished the immunoreactivity of kappa and lambda light chains, and therefore total plasma cells were unable to be counted. Effects of the fixation and en bloc heat-treatment on the reactivity of specific antibodies were evaluated as follows. The detectability of antigen-specific plasma cells was evaluated with a 5-step scale, in comparison with those in 4% PFA-fixed frozen lymph nodes: −: none, +/−: < 5%, +: < 30%, ++: < 60%, and +++: nearly comparable with the control frozen sections.
Statistical analysisStatistical analysis was performed using GraphPad PRISM software (version 5.04, GraphPad software, San Diego, CA, USA). P < 0.05 was regarded as statistically significant.
Factors inactivating the antigen-binding reactivity of antibodies during the FFPE preparation were investigated. As shown in Figure 2A, plasma cells producing antibodies against HRP and those immunoreactive for kappa and lambda light chains were richly distributed in sections of five 4% PFA-fixed lymph nodes of HRP-immunized rats (n = 3); the ratios ranging from 16.7% to 60.4%, with the mean 45.9% and the median 48.0%. During the preparation of FFPE sections, the detectability of plasma cells producing anti-HRP antibodies was decreased, whereas plasma cells immunoreactive for kappa and lambda light chains were kept in number, as illustrated in Figure 2B. The proportion of plasma cells producing antibodies against HRP in total plasma cells was significantly decreased after fixation in unbuffered 10% formalin (the mean 26.4% and median 25.6%), when compared with PFA-fixed frozen sections (the mean 52.3% and median 50.6%), as shown in Figure 2C. En bloc heating of formalin-fixed tissue at 60°C in deionized water tended to show a mild retrieval effect on the antibody reactivity. The detectability of anti-HRP antibody-producing cells after en bloc heating at 60°C was 68.4 ± 2.1% (mean ± SD). The steps of i) ethanol dehydration and ii) xylene penetration did not affect the antibody activity, when compared with iii) unbuffered 10% formalin-fixed frozen tissue sections. The detectability of anti-HRP antibody-producing cells (mean ± SD) was 32.7 ± 4.2% (i), 47.8 ± 12.4% (ii) and 51.1 ± 11.7% (iii), respectively. In FFPE sections, the number of plasma cells producing antibodies against HRP was significantly decreased, when compared with unbuffered 10% formalin-fixed frozen tissue sections (Fig. 2D). The detectability of anti-HRP antibody-producing cells was as low as 1.5 ± 0.6% (mean ± SD).

Effects of the process of preparing FFPE sections on antigen-binding reactivity of antibodies. (A) Consecutive frozen sections of 4% PFA-prefixed lymph nodes of the HRP-immunized rat were stained with HRP and cocktail monoclonal antibodies against kappa and lambda light chains. Anti-HRP antibody-producing plasma cells and total antibody-producing plasma cells were stained brown, respectively. The alphabets in white circles correspond to the lymph node shown in panel B. Bar = 50 μm. (B) Consecutive frozen and paraffin-embedded sections of the lymph node in each step of FFPE processing. Anti-HRP antibody-producing cells and total antibody-producing cells were stained brown with HRP and cocktail monoclonal antibodies against kappa and lambda chains, respectively. The alphabets in white circles correspond to the treatment step shown in Figure 1. (C) The proportion of anti-HRP antibody-producing cells in total plasma cells in 4% PFA-frozen sections and unbuffered 10% formalin-fixed frozen sections. The data are shown as the mean ± SD (n = 3). * means a significant difference (p < 0.05, with a paired samples T test). (D) The detectability of anti-HRP antibody-producing cells in sections of the lymph node in each step of FFPE processing. The data are shown as the mean ± SD (n = 3). ** means a significant difference (p < 0.01, vs. unbuffered formalin-fixed frozen section: with Dunnett test).
Plasma cells producing antibodies against HRP were consistently detectable in frozen sections of the lymph node fixed in 4% PFA (Fig. 3A). As illustrated in Figure 3B, plasma cells immunoreactive for kappa and lambda light chains were abundant in frozen sections of the lymph node fixed in unbuffered 10% formalin for 4, 24, and 48 hr or for one week. Plasma cells producing antibodies against HRP were detected in frozen sections of the lymph node, but the detectability was significantly decreased after fixation in unbuffered 10% formalin for 24 and 48 hr, when compared with the specimens fixed for 4 hr (Fig. 3C). Fixation in unbuffered 10% formalin for one week severely deteriorated the signals. The detectability of anti-HRP antibody-producing cells (mean ± SD) was: 88.7 ± 7.0% (4 hr), 51.1 ± 11.7% (24 hr), 45.1 ± 18.4% (48 hr), and 13.9 ± 6.7% (one week), respectively. The signal intensity was also weakened according to the length of the fixation period.

Effects of formalin-fixation period on the antibody reactivity. (A) Consecutive frozen sections of 4% PFA-fixed lymph nodes of the HRP-immunized rat stained with HRP and cocktail monoclonal antibodies against kappa and lambda light chains. Plasma cells producing anti-HRP antibodies and all the plasma cells were demonstrated in each section. Bar = 50 μm. The alphabets in white circles correspond to the lymph node shown in panel B. (B) Consecutive frozen sections of the lymph node of the HRP-immunized rats fixed in unbuffered 10% formalin for 4, 24 and 48 hr and one week. By using HRP as a probe, anti-HRP antibody-producing cells were seen in frozen sections of the lymph node fixed at room temperature for 4 to 48 hr. Total antibody-producing plasma cells were visualized in each section immunostained with cocktail monoclonal antibodies against kappa and lambda light chains. Bar = 50 μm. (C) The detectability of anti-HRP antibody-producing cells in frozen sections of the lymph node soaked in formalin for 4, 24, and 48 hr and one week. The data are shown as the mean ± SD (n = 3). Significant differences are detected: *p < 0.05, **p < 0.01, ***p < 0.001, with one-way ANOVA and Turkey’s test.
The detectability of plasma cells producing antibodies against HRP in the lymph node was compared among sections fixed for 24 hr in 4% PFA, buffered 10% formalin and unbuffered 10% formalin. As displayed in Figure 4A and 4B, the positively signalized plasma cells were more frequently detected in frozen sections fixed in 4% PFA and buffered 10% formalin than those fixed in unbuffered 10% formalin. The detectability of anti-HRP antibody-producing cells in frozen sections (mean ± SD) was 88.1 ± 20.3% (PFA), 96.7 ± 3.8% (buffered formalin) and 51.1 ± 11.7% (unbuffered formalin), respectively. In paraffin-embedded sections, positive signals were scarcely observed after soaking in each fixative (Fig. 4A). It is noteworthy that slightly better detectability of anti-HRP antibody-producing cells was recognized in paraffin sections after 4% PFA-fixation than after fixation in buffered or unbuffered 10% formalin. The detectability of anti-HRP antibody-producing cells in paraffin sections (mean ± SD) was very low: 3.8 ± 2.5% (PFA), 1.3 ± 0.7% (buffered formalin) and 1.5 ± 0.6% (unbuffered formalin).

Effects of formaldehyde-based fixatives on the anti-HRP antibody reactivity in frozen sections and paraffin-embedded sections of the lymph node sampled from the HRP-immunized rat. (A) Comparative staining with HRP and cocktail monoclonal antibodies against kappa and lambda chains, using consecutive frozen sections and paraffin-embedded sections of the lymph node of HRP-immunized rats fixed in 4% PFA and buffered or unbuffered 10% formalin. Anti-HRP antibody-producing cells were seen in frozen sections of the respective lymph node. Almost no signals were seen in paraffin-embedded sections. Bar = 50 μm. (B) The detectability of anti-HRP antibody-producing cells in frozen sections of the lymph node fixed in 4% PFA and buffered or unbuffered 10% formalin at room temperature for 24 hr. The data are shown as the mean ± SD (n = 3). * means a significant difference (p < 0.05: with one-way ANOVA and Turkey’s test).
Other kinds of fixatives were evaluated for the preservation of the antigen-binding reactivity of the antibodies. These included PLP solution, Zamboni solution and carbodiimide solution as cross-linking fixatives, and ethanol, acetone and Carnoy’s solution as precipitation fixatives. AMeX and PLP-AMeX methods were also examined. As shown in Figure 5 and Tables 1 and 2, the anti-HRP antibody reactivity was relatively well kept in frozen sections of the lymph node fixed in the above fixatives, except for Carnoy’s solution. In ethanol-fixed, acetone-fixed, Zamboni-fixed, and AMeX-treated lymph nodes, the antibody reactivity was preserved after paraffin embedding. Paraffin embedding abolished anti-HRP activity after fixation in carbodiimide. In paraffin sections of Zamboni-fixed, PLP-fixed and PLP-AMeX-treated lymph nodes, anti-HRP activity was detectable, but with significantly decreased reactivity when compared with frozen sections of tissues fixed in the corresponding fixatives. Fixation in ethanol and acetone, as well as the use of the AMeX method, were valuable in applying the enzyme-labeled antigen method to paraffin sections of the lymph node of HRP-immunized rats.

Effects of varied fixatives on the anti-HRP antibody reactivity. Frozen sections and paraffin-embedded sections of the lymph node of the HRP-immunized rats soaked in varied fixatives were stained with HRP. Anti-HRP antibody-producing cells were seen in respective frozen sections, except for the lymph node fixed in Carnoy’s solution. In paraffin-embedded sections of the lymph node fixed in or treated with PLP, PLP-AMeX method, ethanol, acetone and AMeX method, positive signals were visible. In paraffin sections prepared from tissues fixed in carbodiimide and Carnoy’s solutions, positive signals were scarcely observed. Bar = 50 μm.
| Antigen probe | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRP | KLH | OA | BSA | ||||||||||
| Lymph node | Lymph node | Lymph node | Lymph node | ||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Condition | PLP | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ |
| PLP-AMeX | ++ | ++ | ++ | +++ | +++ | +++ | ++ | ++ | +++ | +++ | +++ | +++ | |
| Zamboni | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ | |
| Carbodiimide | ++ | + | +++ | − | − | − | +/− | +/− | +/− | − | − | +/− | |
| Ethanol | +++ | ++ | +++ | + | ++ | + | ++ | +++ | + | ++ | + | + | |
| Acetone | ++ | ++ | +++ | +++ | + | +++ | +++ | +++ | +++ | ++ | ++ | +++ | |
| Carnoy | +/− | + | +/− | +/− | − | − | +/− | +/− | +/− | − | − | − | |
| AMeX | +++ | ++ | +++ | ++ | + | + | ++ | + | +/− | ++ | + | + | |
Rats were immunized with HRP and the mixture of KLH, OA and BSA. The reactivity was evaluated in comparison with that of 4% PFA-fixed frozen sections of the corresponding lymph node. −: none, +/−: < 5%, +: < 30%, ++: < 60%, +++: nearly comparable with the frozen sections.
| Antigen probe | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRP | KLH | OA | BSA | ||||||||||
| Lymph node | Lymph node | Lymph node | Lymph node | ||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Condition | PLP | + | ++ | ++ | + | + | + | + | + | + | + | +/− | +/− |
| PLP-AMeX | + | + | ++ | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | |
| Zamboni | ++ | ++ | ++ | +/− | + | + | +/− | +/− | + | − | +/− | +/− | |
| Carbodiimide | +/− | − | − | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| Ethanol | ++ | +++ | +++ | + | ++ | ++ | ++ | ++ | +++ | + | ++ | + | |
| Acetone | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Carnoy | − | − | − | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| AMeX | +++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | |
Rats were immunized with HRP and the mixture of KLH, OA and BSA. The reactivity was evaluated in comparison with that of 4% PFA-fixed frozen sections of the corresponding lymph node. −: none, +/−: < 5%, +: < 30%, ++: < 60%, +++: nearly comparable with the frozen sections. NT: Not tested.
The same strategy was applied to detecting anti-KLH, anti-OA and anti-BSA antibodies in frozen and paraffin-embedded sections of the lymph node. As shown in Figure 6 and Tables 1 and 2, paraffin embedding of PLP-fixed, Zamboni-fixed and PLP-AMeX-treated lymph nodes abolished the antibody reactivity. The antibody reactivity scarcely remained in frozen sections of carbodiimide-fixed and Carnoy-fixed lymph nodes. It is noteworthy that paraffin sections of acetone-fixed and AMeX-treated lymph nodes preserved positive signals of the antibody reactivity equally to or even better than frozen sections, where the sections were pretreated with 1 μg/ml proteinase K at room temperature for 15 min. In paraffin sections after ethanol fixation, the antibody reactivity was also relatively well kept. In paraffin sections of ethanol-fixed, acetone-fixed and AMeX-treated lymph nodes, positive signals of the antibody reactivity were detectable without proteinase K pretreatment.

Demonstration of plasma cells producing antibodies against KLH, OA and BSA in frozen and paraffin-embedded sections of the lymph node of rats immunized with the mixture of KLH, OA and BSA. The nodes were fixed in various fixatives, and specific antibodies were demonstrated with biotinylated antigen probes. Plasma cells producing anti-KLH, anti-OA and anti-BSA antibodies were visualized in frozen sections of tissues fixed in the respective fixatives. In paraffin-embedded sections of tissues treated with PLP, PLP-AMeX and Zamboni solution, positive signals were significantly decreased, when compared with the corresponding frozen sections. In contrast, in paraffin-embedded sections of tissues treated with ethanol, acetone and AMeX method, positive signals were well retained. Bar = 50 μm.
We further evaluated the antibody reactivity in ethanol-fixed, acetone-fixed and AMeX-treated paraffin-embedded lymph nodes stored for around five years at room temperature. When the paraffin sections of the lymph node were stained with the corresponding immunized antigens, sufficient positive signals were detected in the respective sections, as shown in Figure 7.
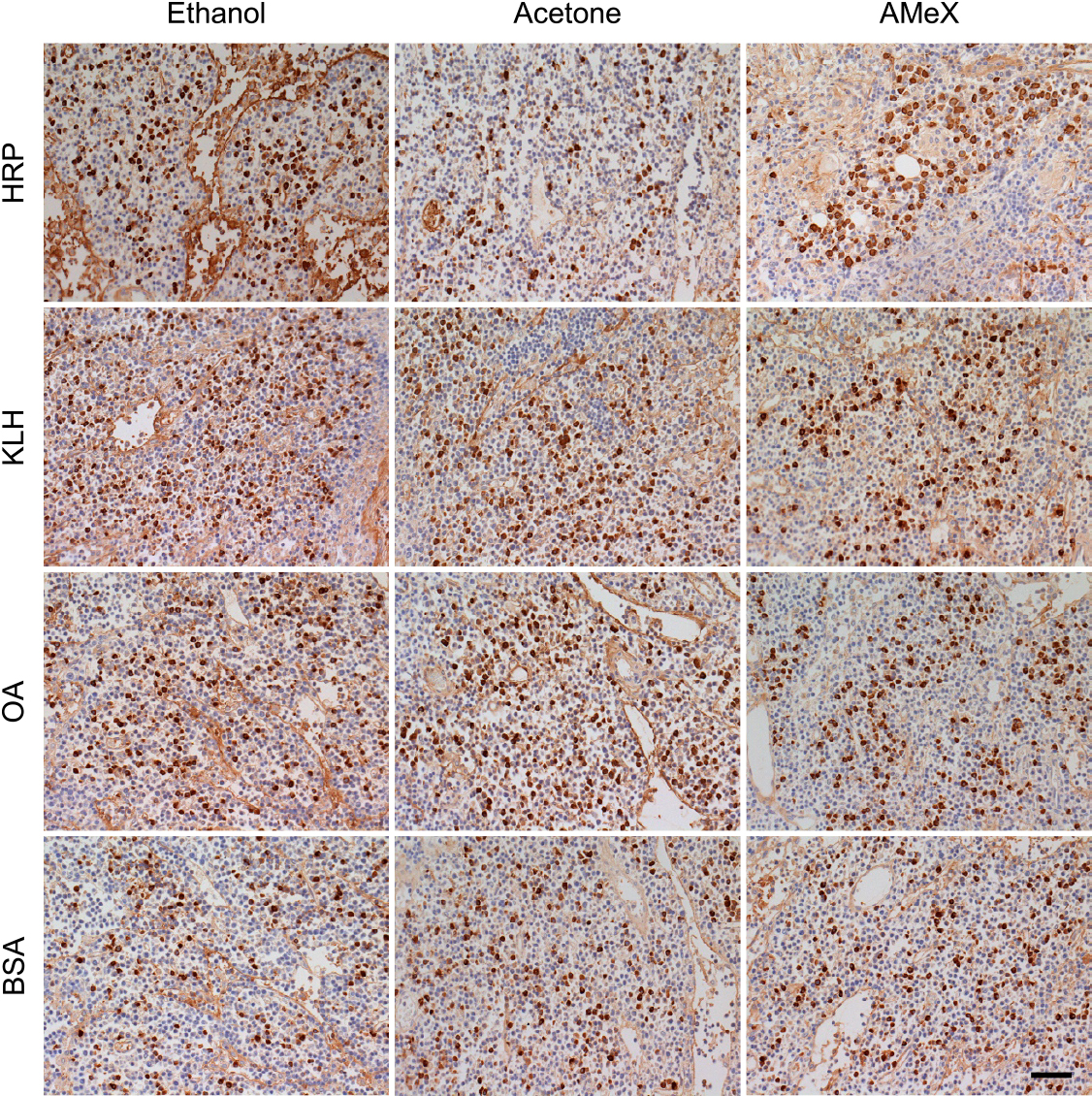
Long-term preservation of the antibody reactivity in paraffin sections prepared from ethanol-fixed, acetone-fixed or AMeX-treated lymph nodes of specific antigen-immunized rats. The blocks were stored at room temperature for five years. With the enzyme-labeled antigen method using antigen probes, plasma cells producing antigen-specific antibodies were visualized with the corresponding antigen probes. Bar = 50 μm.
In frozen sections of unbuffered 10% formalin-fixed lymph nodes of HRP-immunized rats en bloc heated at 60°C, the anti-HRP antibody reactivity trended to recover, as shown in Figure 2B and 2C. Therefore, the effect on the recovery of the anti-HRP antibody reactivity by en bloc heating at 70–90°C was evaluated in unbuffered 10% formalin-fixed lymph nodes of HRP-immunized rats. In both frozen and paraffin-embedded sections of 70°C en bloc-heated lymph nodes of the immunized rats, anti-HRP antibody reactivity was not significantly altered. Whether or not the antibody reactivity was retrieved by en bloc heating in frozen sections prepared from FFPE tissues was also evaluated. The enzyme-labeled antigen method was applied to frozen sections prepared from the lymph node of rats after formalin fixation and FFPE. Plasma cells producing antibodies against HRP were detectable in frozen sections prepared from the unbuffered formalin-fixed lymph node of the HRP-immunized rat. The reactivity was not affected by en bloc heating of the deparaffinized (rehydrated) FFPE tissue at 70°C. In both non-heated and 70°C-heated unbuffered formalin-fixed lymph nodes, the reactivity had significantly been suppressed by paraffin embedding. In both frozen and paraffin sections of unbuffered formalin-fixed lymph nodes heated en bloc at 80°C and 90°C, the antibody reactivity was totally abolished. The results were summarized in Figure 8 and Table 3.

Reactivity of specific antibodies in frozen sections comparatively prepared from the lymph node fixed in unbuffered 10% formalin and those deparaffinized from FFPE tissue with or without en bloc heating. Frozen sections of the lymph node of the HRP-immunized rat, fixed in unbuffered 10% formalin, were stained with HRP as a probe of the enzyme-labeled antigen method. In comparison, FFPE blocks were deparaffinized (hydrated) to prepare frozen sections. Pieces of the tissues were heated en bloc at 70°C in PBS. Anti-HRP antibody-producing cells were detectable in frozen sections of non-heated and heated fixed lymph nodes, but little signals were seen in paraffin sections of the same tissues. In frozen sections prepared from deparaffinized FFPE lymph nodes with or without en bloc heating at 70°C, positive cells were scarcely detected. No positive signals were seen after en bloc heating at 80°C and 90°C. Bar = 50 μm.
| Lymph node | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Condition | Frozen sections | Non-heated | +++ | ++ | ++ |
| 70°C | +++ | ++ | + | ||
| 80°C | − | − | − | ||
| 90°C | − | − | − | ||
| Paraffin-embedded sections | Non-heated | +/− | +/− | +/− | |
| 70°C | +/− | +/− | +/− | ||
| 80°C | − | − | − | ||
| 90°C | − | − | − | ||
| Frozen sections prepared after deparaffinization of FFPE tissues | Non-heated | +/− | +/− | +/− | |
| 70°C | +/− | +/− | +/− | ||
| 80°C | − | − | − | ||
| 90°C | − | − | − | ||
Unbuffered 10% formalin-fixed frozen tissue, FFPE tissue and deparaffinized (hydrated) frozen tissue prepared from FFPE specimens were compared. HRP was used as a probe. The reactivity was evaluated in comparison with that of 4% PFA-fixed frozen sections of the corresponding lymph node. −: none, +/−: < 5%, +: < 30%, ++: < 60%, +++: nearly comparable with the frozen sections.
We evaluated the effect of proteinase K concentration in retrieving the reactivity of antibodies in paraffin-embedded sections after fixation in 4% PFA and buffered or unbuffered 10% formalin. As shown in Figure 9A, the treatment with 20 μg/ml or higher concentrations of proteinase K evidently increased positive signals in 4% PFA-fixed tissue, when compared to 1 μg/ml proteinase K treatment. The detectability of anti-HRP antibody-producing cells in PFA-fixed paraffin-embedded sections treated with 1 to 320 μg/ml proteinase K (mean ± SD) was 6.74 ± 6.80% (1 μg/ml), 68.28 ± 18.84% (20 μg/ml), 77.69 ± 17.90% (80 μg/ml) and 83.21 ± 23.08% (320 μg/ml), respectively. In tissues fixed in buffered or unbuffered 10% formalin, the higher concentration of proteinase K treatment, the more positive signals, but the effect was milder than in 4% PFA-fixed tissue. The detectability of anti-HRP antibody-producing cells in FFPE sections after treatment with 1 to 320 μg/ml proteinase K (mean ± SD) was calculated by comparing with those seen in PFA-fixed frozen sections of the respective lymph node. The values were 0.66 ± 0.87% (1 μg/ml), 21.78 ± 14.42% (20 μg/ml), 45.33 ± 15.66% (80 μg/ml) and 42.97 ± 9.89% (320 μg/ml), respectively. The detectability values of anti-HRP antibody-producing cells in buffered formalin-fixed paraffin-embedded sections after treatment with 1 to 320 μg/ml proteinase K (mean ± SD) were 1.55 ± 2.69% (1 μg/ml), 26.70 ± 3.67% (20 μg/ml), 52.70 ± 20.16% (80 μg/ml) and 55.40 ± 27.65% (320 μg/ml), respectively.

Effects of proteinase K treatment on the anti-HRP reactivity of antibodies in paraffin-embedded sections of the lymph node of HRP-immunized rats, fixed in 4% PFA, unbuffered 10% formalin and buffered 10% formalin. (A) PFA-fixed, unbuffered 10% formalin-fixed and buffered 10% formalin-fixed paraffin-embedded sections after treatment with proteinase K (1, 20, 80 and 320 μg/ml) and incubation with HRP. Proteinase K treatment with 20 μg/ml or higher concentrations evidently enhanced the anti-HRP reactivity in 4% PFA-fixed tissues. In tissues fixed in buffered 10% formalin, proteinase K at high concentrations enhanced the anti-HRP reactivity, but scarcely enhanced in tissues fixed in unbuffered 10% formalin. The degree of enhancement of the anti-HRP reactivity was weaker in tissues fixed in buffered formalin than in those fixed in PFA. Bar = 50 μm. (B–D) The detectability of anti-HRP antibody-producing cells after proteinase K treatment for paraffin-embedded sections of the lymph node fixed in PFA (B), unbuffered 10% formalin (C) and buffered 10% formalin (D). The data represent a percentage of the positive cells in paraffin sections, when compared with 4% PFA-fixed frozen sections, and are shown as the mean ± SD (n = 3). Significant differences are detected: *p < 0.05, **p < 0.01, ***p < 0.001, with one-way repeated measures ANOVA and Turkey’s test.
Hydrated heating of FFPE sections in a pressure cooker was ineffective. After hydrated heating, the remaining antibody reactivity in FFPE sections was totally abolished. The negative effect was independent of the solutions of choice.
Effects of periodate treatment on the retrieval of the antibody reactivity in FFPE sectionsPeriodic acid treatment was ineffective. Combinations of the periodate treatment and heating or proteinase K treatments did not show any effect either (Supplementary Fig. S1).
Effects of antigen concentrations on detecting specific antibodies in FFPE sections of the lymph node of HRP-immunized ratsThe effect of antigen concentrations in detecting the antibody reactivity was evaluated using paraffin-embedded sections of 4% PFA-fixed and buffered or unbuffered 10% formalin-fixed lymph nodes. The higher the concentration of HRP, the more positive signals, as shown in Figure 10. Proteinase K pretreatment did not change the situation. In unbuffered 10% formalin-fixed tissue, the degree of signal amplification was weaker than in 4% PFA-fixed and buffered 10% formalin-fixed tissue.

Effects of probe concentrations on the anti-HRP reactivity of antibodies in paraffin-embedded sections prepared from the lymph node of HRP-immunized rats fixed in 4% PFA, buffered 10% formalin and unbuffered 10% formalin. Consecutive sections of the lymph nodes were incubated with HRP at the concentration of 1, 10 and 100 μg/ml. The number of anti-HRP antibody-producing plasma cells were increased, depending upon the probe concentration. Bar = 50 μm.
The amino acid polymer method, ABC method and CSA-II method were applied to sections of unbuffered 10% formalin-fixed paraffin-embedded lymph nodes of rats immunized with the mixture of KLH, OA and BSA. As shown in Figure 11, with the use of the amplification methods, the positive signals of antibodies against KLH and OA were more strongly detected than those using HRP-labeled streptavidin. However, the signals of anti-BSA antibody were scarcely detected after the use of the amplification methods. Background staining was weaker in the amino-acid polymer method, when compared with the ABC method and CSA-II method: In the ABC and CSA-II methods, background staining was increased. We attempted to reduce the background staining of the ABC and CSA-II methods for detecting signals of anti-KLH antibodies: In the ABC method, the dilution of anti-biotin antibody and/or biotinylated anti-rabbit IgG antibody was not effective to reduce the background staining: Specific positive signals were weakened, as illustrated in Supplementary Figure S2. In case of CSA-II, the secondary reagent of CSA-II was diluted, but the positive signals were also weakened along with staining in the background, as shown in Supplementary Figure S3.

Detection of antigen-specific antibody-producing plasma cells by using signal amplification techniques in paraffin-embedded sections of the lymph node of rats immunized with the mixture of KLH, OA and BSA. The tissue was fixed in unbuffered 10% formalin. Positive signals of anti-KLH and anti-OA antibodies were amplified by the use of amino acid polymer method, ABC method and CSA-II method. Compare them with the standard method using HRP-labeled streptavidin. No anti-BSA antibody producing cells were seen with the use of any methods. Bar = 50 μm.
The enzyme-labeled antigen method was first reported in 1968 independently by Leduc et al. [5] and Straus [21] to visualize plasma cells producing anti-HRP antibodies in frozen sections of the HRP-immunized lymph node. It was just two years after the original report of the enzyme-labeled antibody method by Nakane and Pierce [11]. The method “the enzyme-labeled antigen method” has long been neglected for clinicopathological application, in sharp contrast the extensive development and prevalence of the enzyme-labeled antibody method or immunostaining. We have recruited this historical and intriguing immunohistochemical technique to evaluate the pathogenesis of infectious and autoimmune disorders by using PFA-fixed frozen tissue sections [8, 10, 12, 26]. One of the reasons why the enzyme-labeled antigen method has not been popular is the difficulty in applying the method to FFPE sections. It should be noted that in early 1980’s, the application of the immunostaining to FFPE sections were not yet popular [27].
In the current study, we investigated causes of inactivation of the antibody reactivity in the process of preparing FFPE sections. Experimentally, we first evaluated the lymph node of rats immunized with HRP. When compared with 4% PFA-fixed frozen sections, unbuffered 10% formalin-fixed frozen sections demonstrated a lower proportion of plasma cells producing anti-HRP antibodies among total plasma cells. Ethanol dehydration and xylene penetration following formalin fixation little affected the detectability of anti-HRP reactivity of antibodies. However, the anti-HRP reactivity was significantly abolished by paraffin embedding (Fig. 2). These findings suggested that formalin fixation and paraffin embedding comprised major causes of inactivation of the antibody reactivity in the process of preparing FFPE tissue.
The effect of the fixation period in unbuffered 10% formalin on the antibody reactivity was evaluated using frozen sections of the lymph node of HRP-immunized rats. The detectability of plasma cells producing anti-HRP antibodies was significantly decreased by formalin fixation for 24 or 48 hr, when compared with 4-hr fixation. Formalin fixation for one week almost totally abolished the antibody reactivity (Fig. 3). The period of formalin fixation should thus be a key factor for keeping the antibody reactivity in performing the labeled antigen method.
We also investigated the effect of formaldehyde-based fixatives on the antibody reactivity. The detectability of plasma cells producing anti-HRP antibodies was compared among frozen sections of the lymph node fixed for 24 hr in 4% PFA and buffered or unbuffered 10% formalin. The lymph node fixed in 4% PFA and buffered 10% formalin showed higher detectability rates than the node fixed in unbuffered 10% formalin (Fig. 4). According to the autonomic oxidation of formaldehyde to formic acid, 10% formalin becomes an acidic solution [25], whereas the pH value was adjusted to a neutral state in 4% PFA and buffered 10% formalin. Frozen sections fixed in Carnoy’s solution, an acidic fixative [2], also showed poor staining for antigen-reactive antibodies (Fig. 5). Acidic pH of the fixative should cause the deterioration of the antibody reactivity.
We further analyzed whether or not the labeled antigen method is applicable to frozen and paraffin sections of tissues fixed in various fixatives. Paraffin-embedded nodal tissues fixed in PFA, buffered or unbuffered formalin, PLP solution, PLP-AMeX method, Zamboni solution and 4% carbodiimide solution showed poor reactivity of anti-HRP antibodies, when compared with frozen sections (Figs. 4A and 5, and Tables 1 and 2). All of them belong to fixatives forming protein-protein cross-linkage [14]. It is well known that in the chromogenic immunostaining, the formation of protein-protein cross-linkage inducing conformational changes of protein antigens results in epitope masking and interferes an antigen-antibody reaction [17]. The fixative-induced cross-linkage formation should hamper the antigen-binding reactivity of antibodies. Ethanol and acetone, belonging to organic solvents, induce precipitation fixation of proteins without formation of protein-protein cross-linkage. In the AMeX method, cold acetone is used as a fixative. In paraffin-embedded sections prepared from tissues fixed in these precipitation fixatives, the antibody reactivity was well kept, as in frozen sections (Fig. 5, and Tables 1 and 2). Paraffin embedding after fixation in the precipitation fixatives should be an adequate choice for the enzyme-labeled antigen method.
The analyses were also performed using the lymph node of rats immunized with the mixture of KLH, OA and BSA. In paraffin-embedded sections of the lymph node after fixation in PLP solution and Zamboni solution, the reactivity of antibodies to KLH, OA and BSA was significantly decreased, when compared with frozen sections (Fig. 6, and Tables 1 and 2). The detectability of anti-KLH, anti-OA and anti-BSA reactivity of antibodies was more severely deteriorated than that of HRP. It is reasonable to suppose that the reaction sensitivity of HRP is higher than the biotinylated antigens, as HRP is an enzyme itself.
It is noteworthy that paraffin-embedded sections of the lymph node fixed in ethanol and acetone or treated with the AMeX method consistently showed the antigen-binding reactivity of antibodies, being comparable with or better than that in the frozen sections. It is noteworthy that the antigen-binding signals were detectable without proteinase K pretreatment in paraffin sections of ethanol-fixed, acetone-fixed and AMeX-treated lymph nodes. Acetone fixation and AMeX treatment particularly well and consistently preserved the antibody activity after paraffin embedding. Namely, precipitation fixatives are much superior to cross-linking fixative in maintaining the antigen-binding reactivity of antibodies in paraffin-embedded sections. Sato et al. reported that the antigenicity had been preserved for more than two years without loss of immunoreactivity in AMeX-treated paraffin-embedded tissue, when kept at 4°C [16]. Tanaka et al. reported that the antigenicity was well preserved in acetone-fixed paraffin-embedded sections kept at 4°C for up to two years [24]. In the current study, we demonstrated that the antigen-binding reactivity of antibodies was well preserved in ethanol and acetone-fixed or AMeX-treated paraffin-embedded lymph nodes long stored at room temperature for five years (Fig. 7).
In the lymph node of HRP-immunized rats, the detectability of anti-HRP reactivity after cross-linking fixation was abolished by paraffin embedding (Fig. 2D). We thus checked whether or not deparaffinization of FFPE tissues was effective to recover the antibody reactivity. However, the antibody reactivity in FFPE lymph nodes was not recovered by deparaffinization of FFPE tissues (Fig. 8 and Table 3). It seemed that paraffin embedding after fixation in unbuffered 10% formalin irreversibly inactivated the antibody reactivity.
Hydrated heating has been applied to the antigen (epitope) retrieval of FFPE tissue sections in chromogenic immunostaining [14, 19]. Sections are typically heated for 10 min in 10 mM citrate buffer pH 6.0 or pH 7.0 and 1 mM ethylenediamine tetraacetic acid (EDTA), pH 8.0 [28]. The heating devices mainly include a microwave oven, pressure cooker and autoclave. We reported that hydrated heating in citrate buffer and EDTA with a pressure cooker was ineffective for retrieving the antigen-binding reactivity of antibodies in FFPE sections [7]. The ineffectiveness was reconfirmed in the current study.
In contrast, when the unbuffered formalin-fixed tissue was heated en bloc at 60°C for 4 hr in deionized water, the antibody reactivity was slightly recovered (Fig. 2B and D). We evaluated whether or not the en bloc heating treatment was effective for retrieving the antibody reactivity in FFPE tissue blocks. When the rehydrated (deparaffinized) tissue was heated at 70°C for 4 hr in PBS, the antibody reactivity was not recovered. Heating tissue blocks at 80°C or 90°C totally abolished the antibody reactivity (Fig. 8 and Table 3).
With the use of proteinase K, we also attempted to retrieve the antigen-binding reactivity of antibodies in paraffin-embedded sections of 4% PFA-fixed and buffered or unbuffered 10% formalin-fixed lymph nodes of the HRP-immunized rat. In 4% PFA-fixed paraffin-embedded sections, pretreatment with proteinase K in higher concentrations significantly improved the antibody reactivity when compared with 1 μg/ml proteinase K, that was adequate for retrieving the reactivity in 4% PFA-prefixed frozen sections (Fig. 9A and 9B). In buffered or unbuffered 10% formalin-fixed tissues, the treatment with higher concentrated proteinase K was mildly effective for retrieving the antigen-binding reactivity of antibodies, but the effect was weaker than that in the 4% PFA-fixed tissue (Fig. 9A, 9C and 9D).
Probe concentration also affected the detectability of antigen-specific antibody-producing cells. As expected, the increase of antigen probe concentrations led to enhance antigen specific antibody signals in 20 μg/ml proteinase K-treated paraffin-embedded sections of 4% PFA-fixed and buffered or unbuffered 10% formalin-fixed lymph nodes of the HRP-immunized rats (Fig. 10). Namely, the combination of an adequate retrieval treatment for the antigen-binding reactivity of antibodies and an adequate probe concentration should be important for the success of the enzyme-labeled antigen method in FFPE sections.
We evaluated the effect of periodic acid treatment on the antigen-binding reactivity of antibodies. The antibody reactivity was not improved significantly. We also evaluated combinations of periodic acid treatment and heating or proteinase K treatments. Unfortunately, these combinations did not enhance the reactivity either (Supplementary Fig. S1).
Signal amplification methods should be useful for detecting specific antibody signals with increased sensitivity of detection. These included the polymer-based method, ABC method, CSA-II method, and proximity ligation assay [1, 3, 15, 20]. We attempted to apply the amino acid polymer method, ABC method and CSA-II method to detecting specific antibody-producing cells in FFPE lymph node sections of rats immunized with the mixture of KLH, OA and BSA. These methods amplified positive signals of anti-KLH antibody and OA antibody, when compared with the method using HRP-labeled streptavidin, but not in case of anti-BSA antibody signals (Fig. 11). Even when the concentration of biotinylated BSA was set higher, no positive signals were seen with any amplification method (Supplementary Fig. S4). Background staining was increased with the ABC and CSA-II methods. The dilution of the reagents of the ABC and CSA-II methods resulted in the reduction of specific signals, along with the lowered background staining, as shown in Supplementary Figures S2 and S3. Background staining was weaker with the amino-acid polymer method than that with the ABC and CSA-II methods (Fig. 11). The staining process of the amino acid polymer method is simpler than that of the other two amplification methods: The polymer method uses only two kinds of antibody reagents, anti-biotin antibody and amino acid polymers conjugated with HRP and anti-rabbit IgG.
In conclusion, formalin fixation and paraffin embedding were the major factors inactivating the antigen-binding reactivity of antibodies in the process of preparing FFPE sections. When tissues were fixed in precipitation fixatives as ethanol and acetone, the antibody reactivity were well maintained in paraffin sections. Among the cross-linking fixatives, PFA, PLP and Zamboni solution may be useful for the enzyme-labeled antigen method using paraffin sections. Paraffin sections after fixing in these fixatives and using the AMeX method can be applied to the enzyme-labeled antigen method. In order to utilize the enzyme-labeled antigen method for the research and diagnostic purpose, fresh clinical materials should be handled with such a way. It should be noted that the storage of paraffin-embedded tissues is much easier than that of frozen tissues. Proteolytic enzymatic treatment was effective for retrieving antigen-binding reactivity of antibodies in paraffin-embedded sections of PFA-fixed tissue, but the retrieving effect was not so effective in FFPE sections. Regrettably enough, the signal amplification methods were not a powerful aid for amplifying the specific signals of the enzyme-labeled antigen method in FFPE sections because of insufficient signal/noise ratio. The amino acid polymer technique can be applied as an enhancing tool. Further meticulous studies are needed to retrieve the antigen-binding reactivity of antibodies in FFPE sections for applying the enzyme-labeled antigen method to the clinicopathological investigation and diagnostic pathology.
The authors have no conflict of interest to disclose.
Each author has participated in the work to take public responsibility for appropriate portions of the content. YM planned the study framework and performed most of the study. KS, TT, and AN supported the technical aspects of the study. KI and MS consistently gave advice and stimulation to the analysis. YT fundamentally designed the study and brushed up the manuscript. All authors read and approved the final manuscript.
Skillful technical assistance by Ms. Yukika Hasegawa, Department of Pathology, and Ms. Mika Maeshima, Center for Joint Research Facilities Support, Fujita Health University, Toyoake, are cordially acknowledged. We would like to thank Ms. Hitomi Sato, a student in Fujita Health University Faculty of Medical Technology, for her assistance in the study. This work was supported by JSPS KAKENHI Grant numbers JP18K07030 and JP15K19063.