Abstract
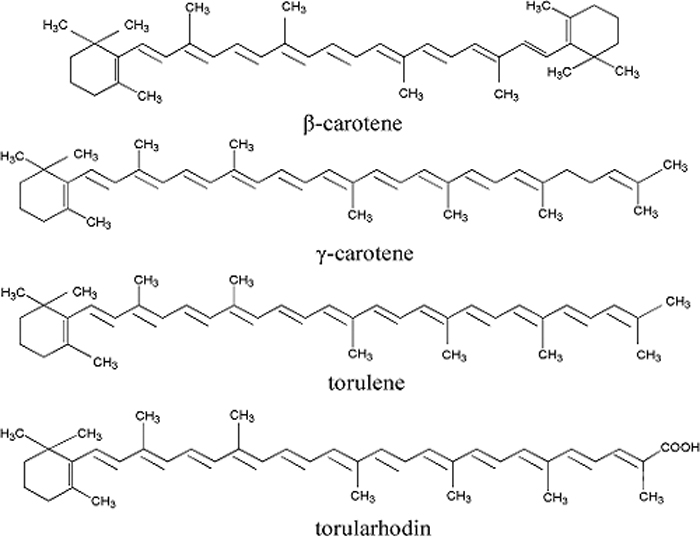
The major carotenoids (β-carotene, γ-carotene, torulene, and torularhodin) were determined by high-performance liquid chromatography, with torulene present in the largest amount (167.0 μg/g), followed by torularhodin (113.4 μg/g), β-carotene (52.1 μg/g) and γ-carotene (15.4 μg/g). In addition, cis/trans torulene isomers were further identified by developing an HPLC-DAD coupled with an atmospheric-pressure chemical ionization (APCI) MS method, following isolation and purification torulene from crude pigments by column chromatography. A total of 8 torulene geometrical isomers were resolved within 60 min by employing a YMC C30 column and a binary gradient mobile phase consisting of methanol–methyl tert–butyl ether–water, (50:47.5:2.5, v/v/v) (A) and methanol–methyl tert–butyl ether–water, (8:90:2, v/v/v) (B). Geometrical carotenoid isomers behave differently with respect to bioavailability; therefore, it is of great importance to expand our knowledge on their biological roles to determine the appropriate method to separate torulene cis/trans isomers.