Abstract
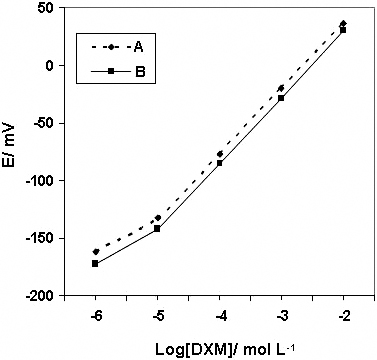
New, simple and convenient potentiometric and spectrophotometric methods are described for the determination of dextromethorphan hydrobromide (DXM) in pharmaceutical preparations. The potentiometric technique is based on developing a potentiometric sensor incorporating the dextromethorphan tetrakis(p-chlorophenyl)borate ion-pair complex as an electroactive species in a plasticized PVC matrix membrane with o-nitophenyl octyl ether or dioctyl phthalate. The sensor shows a rapid near Nernstian response of over 1 × 10−5 – 1 × 10−2 mol L−1 dextromethorphan in the pH range of 3.0 – 9.0. The detection limit is 2 × 10−6 mol L−1 DXM and the response time is instantaneous (2 s). The proposed spectrophotometric technique involves the reaction of DXM with eriochrom black T (EBT) to form an ion-associate complex. Solvent extraction is used to improve the selectivity of the method. The optimal extraction and reaction conditions have been studied, and the analytical characteristics of the method have been obtained. Linearity is obeyed in the range of 7.37 – 73.7 × 10−5 mol L−1 DXM, and the detection limit of the method is 1.29 × 10−5 mol L−1. The relative standard deviation (RSD) and relative error for six replicate measurements of 3.685 × 10−4 mol L−1 are 0.672 and 0.855%, respectively. The interference effect of some excepients has also been tested. The drug contents in pharmaceutical preparations were successfully determined by the proposed methods by applying the standard-addition technique.