Abstract
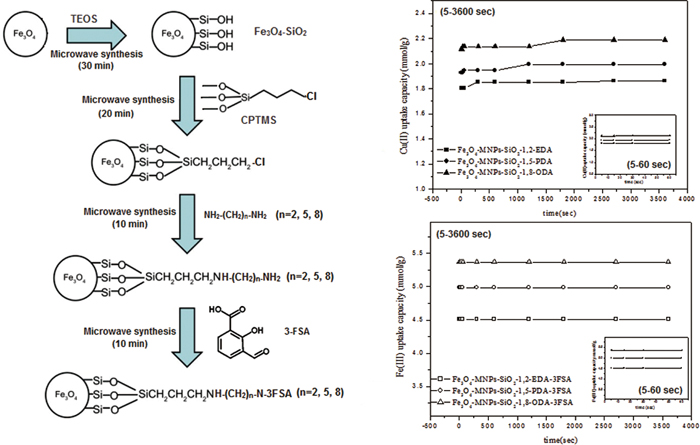
The use of a microwave assisted solvent-free technique for silica coating of iron magnetic nanoparticles (Fe3O4-MNPs) and their functionalization with three aliphatic diamines: 1,2-ethylenediamine (1,2EDA), 1,5-pentanediamine (1,5PDA) and 1.8-octanediamine (1,8-ODA), were successfully achieved in a very short time. Only 60 min were needed for the nano-adsorbent modification as compared with more than 1000 min using conventional methods under reflux conditions. Their surface characteristics (observed by TEM, XRD and FT-IR), in addition to Cu(II) adsorption capacities (1.805, 1.928 and 2.116 mmol g−1) and time of equilibration (5 s) were almost the same. Thus, the time required to accomplish the solid phase extraction process is greatly reduced. On the other hand, the phenomenon of the fast equilibration kinetics was successfully extended on using the functionalized aliphatic diamines magnetic nano-adsorbents as precursors for further microwave treatment. Three selective magnetic nano-adsorbents (Fe3O4-MNPs-SiO2-1,2EDA-3FSA, Fe3O4-MNPs-SiO2-1,5PDA-3FSA and Fe3O4-MNPs-SiO2-1,8ODA-3FSA) were obtained via the reaction with 3-formayl salicylic acid (3FSA) as a selective reagent for Fe(III). At 5 s contact time, they exhibited maximum Fe(III) uptake equal to 4.512, 4.987 and 5.367 mmol g−1, respectively. Furthermore, modeling of values of metal uptake capacity obtained at different shaking time intervals supports pseudo-second order kinetics.