Abstract
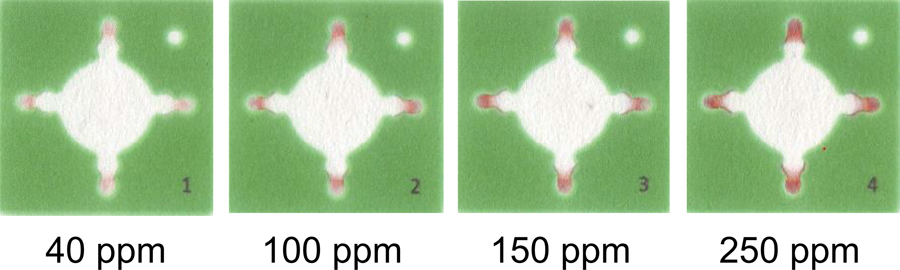
Microfluidic paper-based analytical devices (μPADs) were used to detect the iron ion content in the water of a natural hot spring in order to assess the applicability of this process to the environmental analysis of natural water. The μPADs were fabricated using a wax printer after the addition of hydroxylamine into the detection reservoirs to reduce Fe3+ to Fe2+, 1,10-phenanthroline for the forming of a complex, and poly(acrylic acid) for ion-pair formation with an acetate buffer (pH 4.7). The calibration curve of Fe3+ showed a linearity that ranged from 100 to 1000 ppm in the semi-log plot whereas the color intensity was proportional to the concentration of Fe3+ and ranged from 40 to 350 ppm. The calibration curve represented the daily fluctuation in successive experiments during four days, which indicated that a calibration curve must be constructed for each day. When freshly prepared μPADs were compared with stored ones, no significant difference was found. The μPADs were applied to the determination of Fe3+ in a sample of water from a natural hot spring. Both the accuracy and the precision of the μPAD method were evaluated by comparisons with the results obtained via conventional spectrophotometry. The results of the μPADs were in good agreement with, but less precise than, those obtained via conventional spectrophotometry. Consequently, the μPADs offer advantages that include rapid and miniaturized operation, although the precision was poorer than that of conventional spectrophotometry.