2022 Volume 8 Issue 1 Pages 86-90
2022 Volume 8 Issue 1 Pages 86-90
Abstract
The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial has had a great impact on the management of chronic coronary artery disease (CAD). One of the implications of this trial is the importance of close patient follow-up. To improve patient prognosis, evaluation of the residual extent of ischemia after treatment may be important because several studies have shown a close relationship between residual ischemia and cardiac events. For this assessment, myocardial perfusion single-photon emission computed tomography (MPS) has been utilized and is almost the only modality.
Among the participants in the ISCHEMIA trial, more than 10% were excluded due to the absence of obstructive CAD. The pathophysiology of ischemia without non-obstructive coronary artery disease (INOCA) is gaining recognition; however, diagnosis is difficult, except for the assessment of myocardial flow reserve (MFR). Myocardial perfusion positron emission tomography (PET) is the most common modality for noninvasive evaluation of MFR; however, its availability in Japan is limited. For a breakthrough in this situation, a novel gamma camera with a cadmium zinc telluride (CZT) semiconductor might be one of the solutions that enables the evaluation of MFR with a commercially available perfusion tracer, similar to PET. Another solution is a novel PET tracer with a longer half-life. Clinical trials with 18F labeled perfusion agents have been initiated in Japan, and in a few years, delivery of this perfusion tracer will result in more frequent and easier assessment of MFR.
The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial has had a great impact on the management of patients with chronic coronary artery disease (CAD) (1). The results of the ISCHEMIA trial showed no evidence that the initial invasive strategy reduced the risk of ischemic cardiovascular events or death from any cause over a median of 3.2 years compared to the initial conservative strategy among patients with moderate or severe stable CAD. Therefore, the assessment of the severity of myocardial ischemia with noninvasive imaging modalities seems to be unnecessary, since it does not contribute to the decision of treatment strategy for these patients.
However, the recent revisions or focus updates of guidelines, such as the European Society of Cardiology (ESC) (2) and the Japanese Circulation Society (3), have not eliminated the importance of noninvasive imaging assessments. Moreover, these revised guidelines emphasized the importance of pre-test probability (PTP) and clinical likelihood (CL) and recommended using them for making proper choice of diagnostic imaging modalities.
In this article, we would like to review the implications of ISCHEMIA trial and the role of nuclear cardiology in the post-ISCHEMIA trial era.
Message from the ISCHEMIA trial
The ISCHEMIA trial showed no significant differences between the prognosis of invasive and conservative strategies in patients with moderate-to-severe myocardial ischemia (Figure 1A) (1). The primary outcome of this study was a composite of death from cardiovascular causes, myocardial infarction, hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest; however, revascularization therapy was not included as the primary outcome. More than 20% of the patients in the conservative strategy group received revascularization therapy, including the emergent procedure (Figure 1-B). Moreover, the incidence of nonprocedural myocardial infarction and hospitalization for unstable angina tended to be higher in the conservative strategy group.
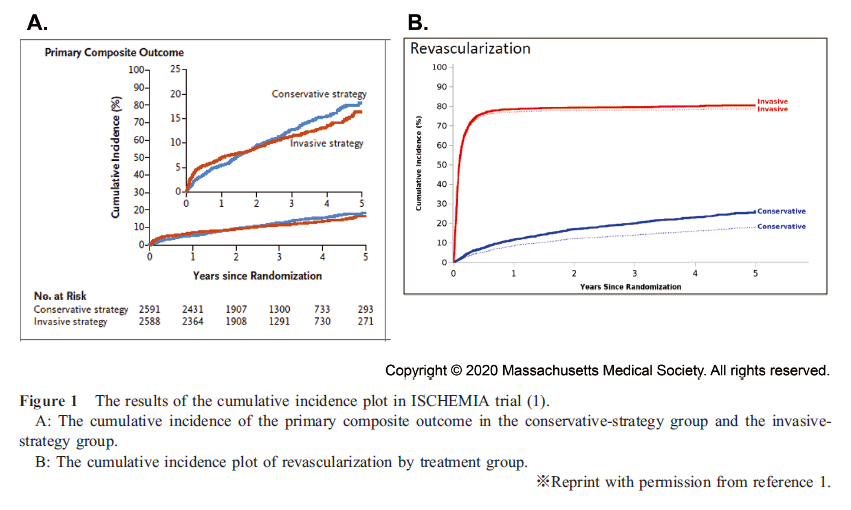
Considering these results, the message of this trial is not that an invasive strategy in the treatment of chronic stable angina is useless but rather that not all patients need revascularization therapy if they receive optimal medical therapy (OMT) and close and strict management.
What are the prognostic factors after treatment?
As mentioned above, one of the major messages of the ISCHEMIA trial is the importance of close patient management. This raises an important question of what index should be considered to improve patient prognosis after therapy.
Similar to the ISCHEMIA trial, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial showed that as an initial management strategy in patients with stable CAD, percutaneous coronary intervention (PCI) did not reduce the risk of major cardiovascular events, when added to OMT (4).
However, after the publication of the COURAGE trial, a nuclear substudy was reported (5). This study showed that in patients who underwent serial myocardial perfusion single-photon emission computed tomography (MPS) imaging, the addition of PCI to OMT increased the reduction of the ischemic region. This study also suggested a treatment target of ≥ 5 % ischemia reduction with OMT with or without coronary revascularization for the reduction of cardiac event risks (Figure 2).
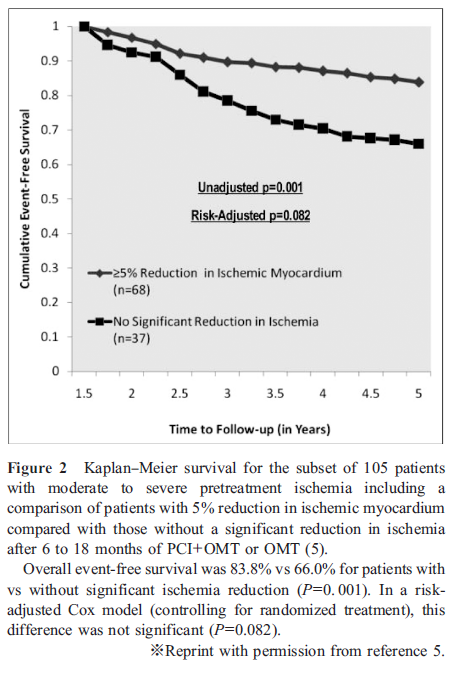
Moreover, a multicenter trial in Japan, named “the Japanese Assessment of Cardiac Events and Survival Study by Quantitative Gated SPECT 4 (J-ACCESS 4),” showed that the incidences of coronary revascularization and cardiac events were significantly higher and lower, respectively in patients with ≥ 5 % ischemia reduction than in those without (6). Moreover, patients without residual ischemia at the time of second MPS had significantly better prognosis. Considering these results, reducing ischemia by ≥ 5% and complete resolution of ischemia could improve prognosis in patients with stable CAD.
The two studies showed similar results despite differences in race and timing. As these results indicate a close relationship between residual ischemia and prognosis, physicians should consider this index for the management of patients with stable CAD after treatment.
MPS might be the only technique among noninvasive imaging modalities, such as computed tomography (CT), magnetic resonance imaging, and echocardiography, that can evaluate the extent of the ischemic region. Therefore, even in the era of post-ISCHEMIA trial, MPS can provide important information for the management of patients with chronic CAD after therapy.
Novel pathophysiology of “INOCA”
In the ISCHEMIA trial, 1,218 patients among a total of 8,518 patients were excluded from the final randomized study due to the lack of evidence of obstructive disease on coronary CT angiography (1). This pathophysiology, known as “ischemia with nonobstructive CAD (INOCA),” has been recognized as a major cause of ischemic heart disease. The European Association of Percutaneous Cardiovascular Interventions, corroborated by the European Society of Cardiology (ESC), documented the expert consensus about this novel concept of INOCA, and explained that the culprit might be a heterogeneous mechanism with coronary microvascular dysfunction (CMD) and vasospastic angina (7). Recently, the incidence of INOCA, especially CMD, has been higher than expected. In the ISCHEMIA study, more than 14% of the total study participants were diagnosed with INOCA, and in another study, more than half of the patients, who were suspected to have CAD but showed normal perfusion by positron emission tomography (PET), showed CMD (8, 9).
However, the diagnosis of CMD is difficult without the evaluation of physiological coronary flow, such as coronary flow reserve and index of microcirculatory resistance by invasive strategies, or myocardial flow reserve (MFR) by PET imaging (9).
Quantitative assessment of MFR is useful for the diagnosis of INOCA as well as the prediction of cardiovascular disease (CVD) risks and therapeutic effect of early revascularization in CAD patients (10, 11).
Based on this evidence, cardiologists should consider physiological blood flow for the management of stable CAD to avoid costly/unnecessary additional testing and better define the disease to develop evidence-based management.
Availability of myocardial perfusion PET in Japan
The usefulness of MFR assessment with PET has been described above; however, its availability in Japan is limited.
13N labeled ammonia is the only agent permitted for health insurance coverage in Japan. PET imaging using this agent is available in only 10 facilities in Japan, where only approximately 200 cases per year are performed (from the annual report of JROAD: The Japanese Registry Of All cardiac and vascular Diseases 2016; Japanese).
One of the reasons for this limitation is the high cost of installation of the on-site cyclotron and automated synthesizer of 13N ammonia and personnel expenses of hiring experts for cyclotron and pharmacological synthesis.
In the USA, more than 250 facilities are available for myocardial perfusion PET imaging. The PET imaging agent utilized most frequently in the USA, 82Rb, is produced by an on-site generator and not a cyclotron. It is much cheaper than 13N ammonia, since capital investment and human costs are not required. Although, the use of 82Rb in Japan is desirable, because of its high import costs, it has limited profitability.
Recently, a high-sensitivity gamma camera equipped with cadmium zinc telluride (CZT) semiconductor detectors has been developed. Two models of the CZT gamma camera are available in Japan, and more than 20 hospitals have already installed them. Its image quality and spatial resolution are superior to those of conventional Anger-type gamma cameras. Moreover, because a high-sensitivity CZT gamma camera consists of multiple detectors, it can simultaneously evaluate whole-heart perfusion. These advances have made quantitative MFR analysis feasible, similar to PET imaging. Several papers have been published about its usefulness in the quantitative analysis of MFR for the diagnosis of multivessel disease (12–15). In contrast to PET imaging, MFR analysis with a CZT semiconductor gamma camera does not require a cyclotron, thereby reducing the installation cost. In the near future, as the numbers of installation sites of CZT semiconductor gamma cameras increase, MFR assessment will be more frequently and easily available.
Another topic of discussion in myocardial perfusion PET imaging is a novel tracer named “18F labeled Flurpiridaz.” In the USA, a phase III clinical trial has been completed, and Flurpiridaz PET myocardial perfusion imaging shows promise as a new tracer for CAD detection and in the assessment of female and obese patients, and those undergoing pharmacological stress testing (16). A second phase III Food and Drug Administration trial is ongoing in the USA. Moreover, clinical trial of this novel tracer in Japan has already begun (17). Since this tracer is an 18F labeled agent, similar to fluorodeoxyglucose (FDG), it can be delivered from pharmaceutical companies, and on-site cyclotron production will not be required. Furthermore, because this agent has a better extract fraction than 13N ammonia and 82Rb, a higher accuracy of MFR assessment is expected (18). In the next few years, as this tracer becomes clinically available in Japan, MFR assessment will be widely used.
Conclusion
In the post-ISCHEMIA trial era, nuclear cardiology should be utilized because of PTP and CL. Furthermore, the importance of assessing residual ischemia for better prognosis in patients with chronic CAD will increase, and the evaluation of MFR for the diagnosis of INOCA as well as obstructive CAD will be essential. For these purposes, nuclear cardiology plays a greater role than other noninvasive imaging modalities.
Acknowledgments
None.
Sources of funding
None.
Conflicts of interest
None.
References
1. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020; 382: 1395–407.
2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–77.
3. Nakano S, Kohsaka S, Chikamori T, Fukushima K, Kobayashi Y, Kozuma K, et al. JCS 2022 Guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J 2022; 86: 882–915.
4. Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503–16.
5. Shaw LJ, Berman DS, Maron DJ, Mancini GBJ, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117: 1283–91.
6. Nanasato M, Matsumoto N, Nakajima K, Chikamori T, Moroi M, Takehana K, et al. Prognostic impact of reducing myocardial ischemia identified using ECG-gated myocardial perfusion SPECT in Japanese patients with coronary artery disease: J-ACCESS 4 study. Int J Cardiol 2018; 267: 202–7.
7. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020; 41: 3504–20.
8. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014; 129: 2518–27.
9. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72: 2625–41.
10. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015; 131: 19–27.
11. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011; 124: 2215–24.
12. Shiraishi S, Sakamoto F, Tsuda N, Yoshida M, Tomiguchi S, Utsunomiya D, et al. Prediction of left main or 3-vessel disease using myocardial perfusion reserve on dynamic thallium-201 single-photon emission computed tomography with a semiconductor gamma camera. Circ J 2015; 79: 623–31.
13. Ben-Haim S, Murthy VL, Breault C, Allie R, Sitek A, Roth N, et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: a feasibility study. J Nucl Med 2013; 54: 873–9.
14. Agostini D, Roule V, Nganoa C, Roth N, Baavour R, Parienti JJ, et al. First validation of myocardial flow reserve assessed by dynamic 99mTc-sestamibi CZT-SPECT camera: head to head comparison with 15O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur J Nucl Med Mol Imaging 2018; 45: 1079–90.
15. Otaki Y, Manabe O, Miller RJH, Manrique A, Nganoa C, Roth N, et al. Quantification of myocardial blood flow by CZT-SPECT with motion correction and comparison with 15O-water PET. J Nucl Cardiol 2021; 28: 1477–86.
16. Maddahi J, Lazewatsky J, Udelson JE, Berman DS, Beanlands RSB, Heller GV, et al. Phase-III clinical trial of Fluorine-18 flurpiridaz positron emission tomography for evaluation of coronary artery disease. J Am Coll Cardiol 2020; 76: 391–401.
17. Kawano M, Tsuchiya J, Bae H, Kimura K, Yokoyama K, Takahashi M, et al. Phase I clinical study of NMB58, a novel positron emission tomography (PET)-myocardial perfusion imaging tracer, conducted to evaluate its safety and pharmacokinetics in Japanese healthy adult males. Ann Nucl Med 2021; 35: 580–8.
18. Maddahi J, Packard RR. Cardiac PET perfusion tracers: current status and future directions. Semin Nucl Med 2014; 44: 333–43.