2023 年 23 巻 2 号 p. 15-19
2023 年 23 巻 2 号 p. 15-19
Purpose: This study investigated the effects of the main ions thought to be released from bioactive dental materials on the proliferation and differentiation of MC3T3-E1 murine osteoblasts.
Methods: Cell proliferation was assessed in a control group (minimum essential medium α) and in several ion groups (control group medium + aluminum, boron, sodium fluoride, or strontium ions) to determine which ions affect the osteogenic response to MC3T3-E1. Three groups of MC3T3-E1 cells, namely, a control group, a lipopolysaccharide (LPS) group (control + 5 μg/mL LPS), and a strontium group (control + 1 mM strontium ions) were cultured for 7 days.
Results: Significant differences in cell proliferation were observed in the strontium group (P < 0.05) but not in the aluminum, boron, or sodium fluoride ion groups. Cell proliferation was enhanced in the strontium group compared with the control group (P < 0.05). Cytotoxicity was not observed in any of the groups (P < 0.05). The strontium group had the highest alkaline phosphatase (ALP) activity (P < 0.05) and the greatest degree of ALP staining, indicating that strontium affected cell differentiation. Collagen production was the highest in the control group and the lowest in the LPS group (P < 0.05).
Conclusion: Media containing 1 mM strontium ions promoted osteoblastic differentiation in MC3T3-E1 cells without causing cytotoxicity. Further research is needed to determine the influence of culture time and the minimum concentration for each ion.
Bioactive dental materials that release ions are widely used in dentistry. These materials release ions such as aluminum (Al), boron (B), fluorine (F), sodium (Na), silicon (Si), and strontium (Sr) and are used in numerous dental materials, including resin composites [1], fissure sealants [2], dentin bonding agents [3], root canal sealers [4], and tooth-coating materials [5]. These ions are thought to have a variety of effects, including caries prevention [6], inhibition of bacterial adhesion [2], suppression of decalcified lesions [7], antibacterial activity, anti-plaque formation activity [1], and acid-buffering capacity [1,8].
Ions released by bioactive dental material can affect various biological phenomena. For example, fluoride ions exert an antimicrobial effect [9] and induce the remineralization of enamel [10], strontium ions are known to enhance bone formation [11], silicate ions are believed to have antioxidant effects and promote bone formation [12], and boron ions stimulate osteogenic and odontogenic differentiation [13]. Consequently, the release of these multiple ions may have a positive effect on the proliferation and differentiation of osteoblasts.
This study investigated the effects of the main ions thought to be released from bioactive dental materials on the proliferation and differentiation of MC3T3-E1 cells.
In this study, experimental ion solutions (Fujifilm Wako Pure Chemical Corp., Osaka, Japan) are prepared following the type and amount of ions released from surface pre-reacted glass-ionomer (S-PRG) filler eluate reported previously [14]. Ions at various concentrations are listed in Table 1.
Cell cultureMouse calvaria-derived MC3T3-E1 cells, which differentiate into osteoblasts, were provided by the RIKEN BioResource Research Center (Tsukuba, Japan). The cells were maintained in minimum essential medium α (MEM α) + l-glutamine + ribonucleosides + deoxy-ribonucleosides (Gibco, Thermo Fisher Scientific Inc., Waltham, MA, USA), containing 1% (v/v) penicillin-streptomycin solution (100 U/mL penicillin and 100 μg/mL streptomycin) and 10% (v/v) fetal bovine serum (FBS; Ireland origin, Biowest SAS, Nuaillé, France). The cells were incubated at 37°C under a humidified atmosphere composed of 95% air and 5% CO2. The medium was changed every other day until the cells had reached 70%-80% confluence, after which the cells were seeded in 96-well plates at 4.0 × 104 cells/well for use in the subsequent cell proliferation, cytotoxicity, alkaline phosphatase (ALP) activity, and collagen quantification experiments.
First, cell proliferation was assessed in the control group (n = 6, MEM α + 10% FBS) and in the several ion (Fujifilm Wako Pure Chemical Corp.) groups (n = 6, control group medium + each ion concentration shown in Table 1) to determine which ions affect MC3T3-E1.
After the cells were treated with the selected ion solution, the cells were seeded again in three groups (n = 6 per group for each experiment): a control group (MEM α + 10% FBS), a lipopolysaccharide (LPS) group (control group medium + 5 μg/mL LPS), and strontium (Fujifilm Wako Pure Chemical Corp.) group (control group medium + 1 mM strontium). The amount of 1 mM strontium was selected because it is thought to be the concentration at which the S-PRG filler releases ions. LPS (Escherichia coli 0111: B4; Sigma-Aldrich Corp., St. Louis, MO, USA) was used to induce inflammation in cells. The concentration of LPS was decided with reference to a previous study [15]. The medium was changed every other day for 7 days prior to assessing the cell proliferation, cytotoxicity, ALP activity, and collagen production.
Cell proliferationCell proliferation in the control group, ion group with a different concentration as shown in Table 1, strontium group, and LPS group was assessed using the Cell Counting Kit-8 (CCK-8) assay (Dojin Laboratories, Kamimashiki, Japan), a well-established method for evaluating cell viability. The cells were incubated for 1 h and the optical density of each well was measured at a wavelength of 450 nm on an iMark microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Table 1 Ions in various concentrations with reference to the dilution of surface pre-reacted glass-ionomer filler eluate
| S-PRG dilute | 1/25,000 | 1/5,000 | 1/1,000 | 1/200 |
|---|---|---|---|---|
| Aluminum (mM) | 0.02 | 0.1 | 0.5 | 2.5 |
| Boron (mM) | 4.00 | 20.0 | 100.0 | 500.0 |
| Strontium (mM) | 0.08 | 0.4 | 2.0 | 10.0 |
| Fluoride (mM) | 0.20 | 1.0 | 5.0 | 25.0 |
Lactate dehydrogenase (LDH) is a useful marker for detecting damage to cell membranes. Cytotoxicity was evaluated using the LDH-Cytotoxicity Test (Fujifilm Wako Pure Chemical Corp.). After adding 100 μL of the test reagent to each well, the samples were incubated at 37°C for 90 min. The optical density of each well was then measured at 570 nm on a microplate reader.
ALP activityALP activity can be used to assess cell differentiation. ALP activity was determined using LabAssay ALP kits (Fujifilm Wako Pure Chemical Corp.). After adding 100 μL of the test reagent to each well, the samples were incubated at 37°C for 15 min. The optical density of each well was then measured at 405 nm on a microplate reader.
Collagen quantificationThe cultured cells were washed with phosphate-buffered saline (Sigma-Aldrich Corp.) and fixed in Bouin solution (Fujifilm Wako Pure Chemical Corp.), then washed again and stained for 1 hr in Sirius Red (Fujifilm Wako Pure Chemical Corp.) dissolved in picric acid solution (Fujifilm Wako Pure Chemical Corp.). Each well was measured at 550 nm on a microplate reader to determine the collagen content.
Statistical analysisAll data are presented as the mean ± standard deviation (n = 6). The distribution and variance of the data were analyzed by the Shapiro-Wilk test and Levene’s test. The cell proliferation and collagen quantification data indicated normal distribution and homogeneity of variance, then both data were analyzed by Tukey’s honestly significant difference test. The ALP activity indicated normal distribution and non-homogeneity of variance, then data was analyzed by Dunnett’s T3 test. Differences in cell proliferation at different concentrations of strontium were analyzed by a t-test with Bonferroni correction, since data indicated normal distribution and homogeneity of variance. A P-value of <0.05 was considered to indicate statistical significance and all analyses were performed using SPSS version 26.0 for Windows, (IBM Corp., Armonk, NY, USA).
Cell proliferation in the ion groups was measured by performing CCK-8 assays after 7 days of incubation. As shown in Fig. 1, a significant difference was observed in only the strontium group (P < 0.05, t-test with Bonferroni correction).
Cell proliferation in the control group, LPS group, and 1-mM strontium group was measured by performing CCK-8 assays after 7 days of incubation. As shown in Fig. 2, cell proliferation was enhanced in the experimental groups (5 μg/mL LPS, 1 mM strontium ions) compared with the control group. Cell proliferation was highest in the strontium group, with a significant difference compared with the control group (P < 0.05, Tukey’s honestly significant difference test).
CytotoxicityLDH was used to monitor the occurrence of cell membrane damage after 7 days of incubation. As shown in Fig. 3, there were no significant differences between the control group, LPS group, and strontium group (P > 0.05, t-test with Bonferroni correction). In addition, no differences in the cell morphology were observed (data not shown). Taken together, these results indicate that none of the treatments exerted cytotoxic effects on the MC3T3-E1 cells.
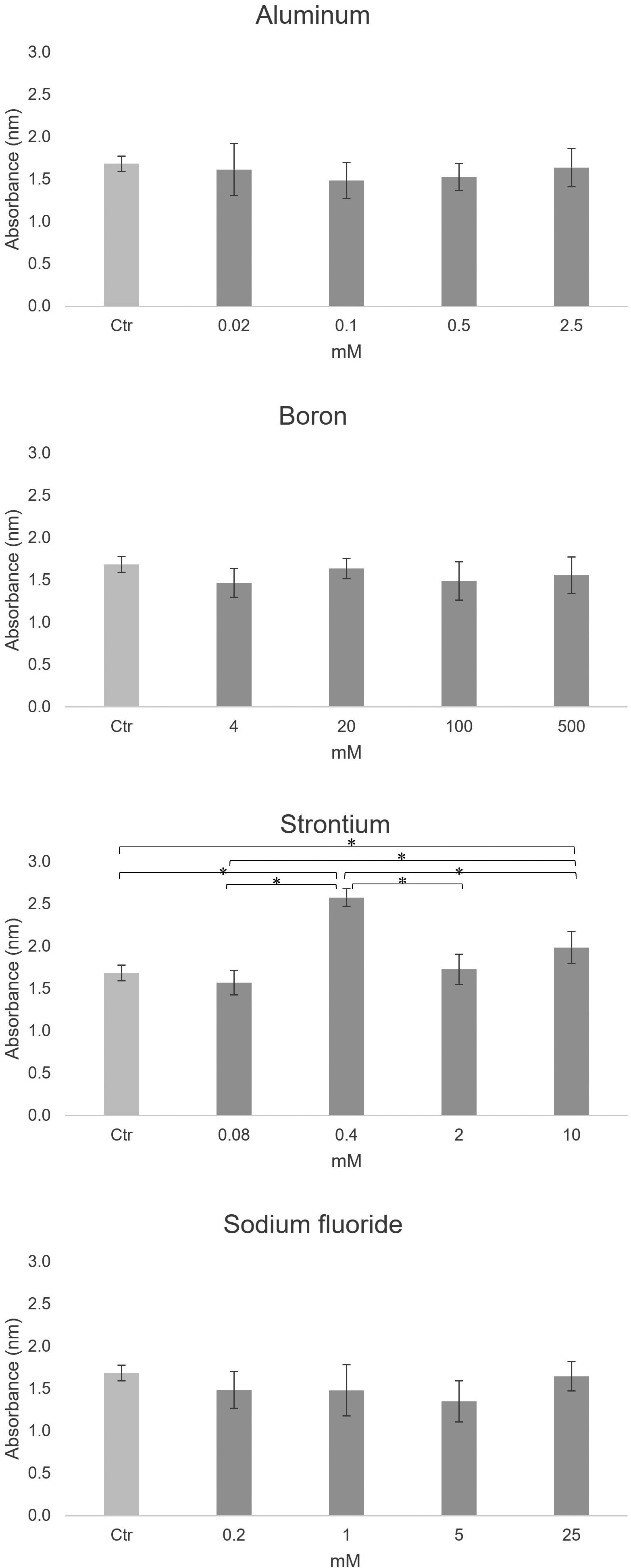
There was a significant difference in only the strontium group (n = 6, *P < 0.05, t-test with Bonferroni correction)
ALP activity was measured using LabAssay ALP kits after 7 days of incubation. As shown in Fig. 4a, significant differences were observed between all groups (P < 0.05, Dunnett’s T3 test). The LPS group displayed the lowest ALP activity, indicating that the cells cultured in the presence of LPS had reduced calcification. As seen from the ALP staining in Fig. 4b, the strontium group stained more deeply than the other groups, showing that the strontium affected cell differentiation.
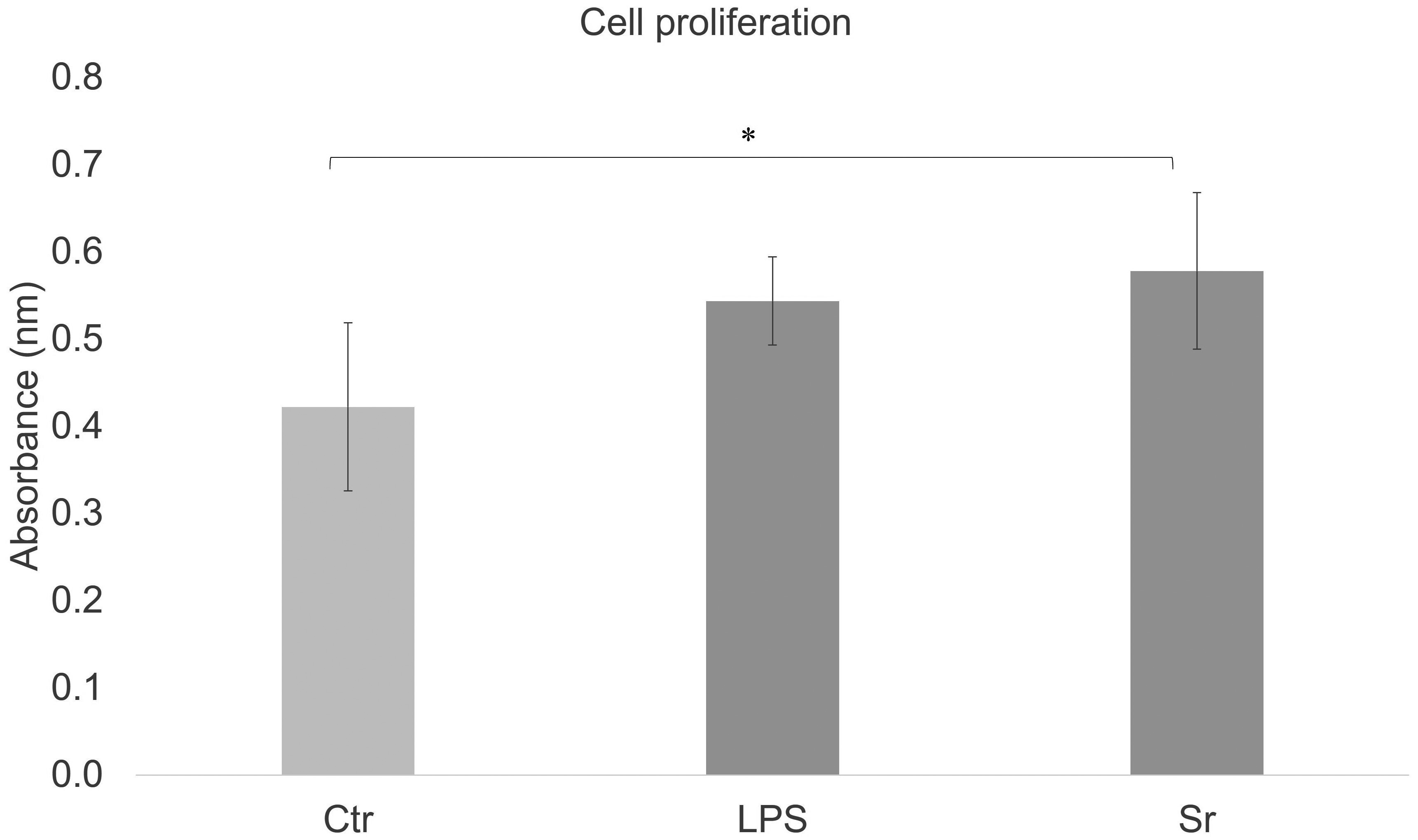
There was a significant difference between the control group and the strontium group (n = 6, *P < 0.05, Tukey’s honestly significant difference test).

There were no significant differences between any of the groups (n = 6, P > 0.05, t-test with Bonferroni correction).

There were significant differences between all of the groups (n = 6, *P < 0.05, Dunnett’s T3 test).

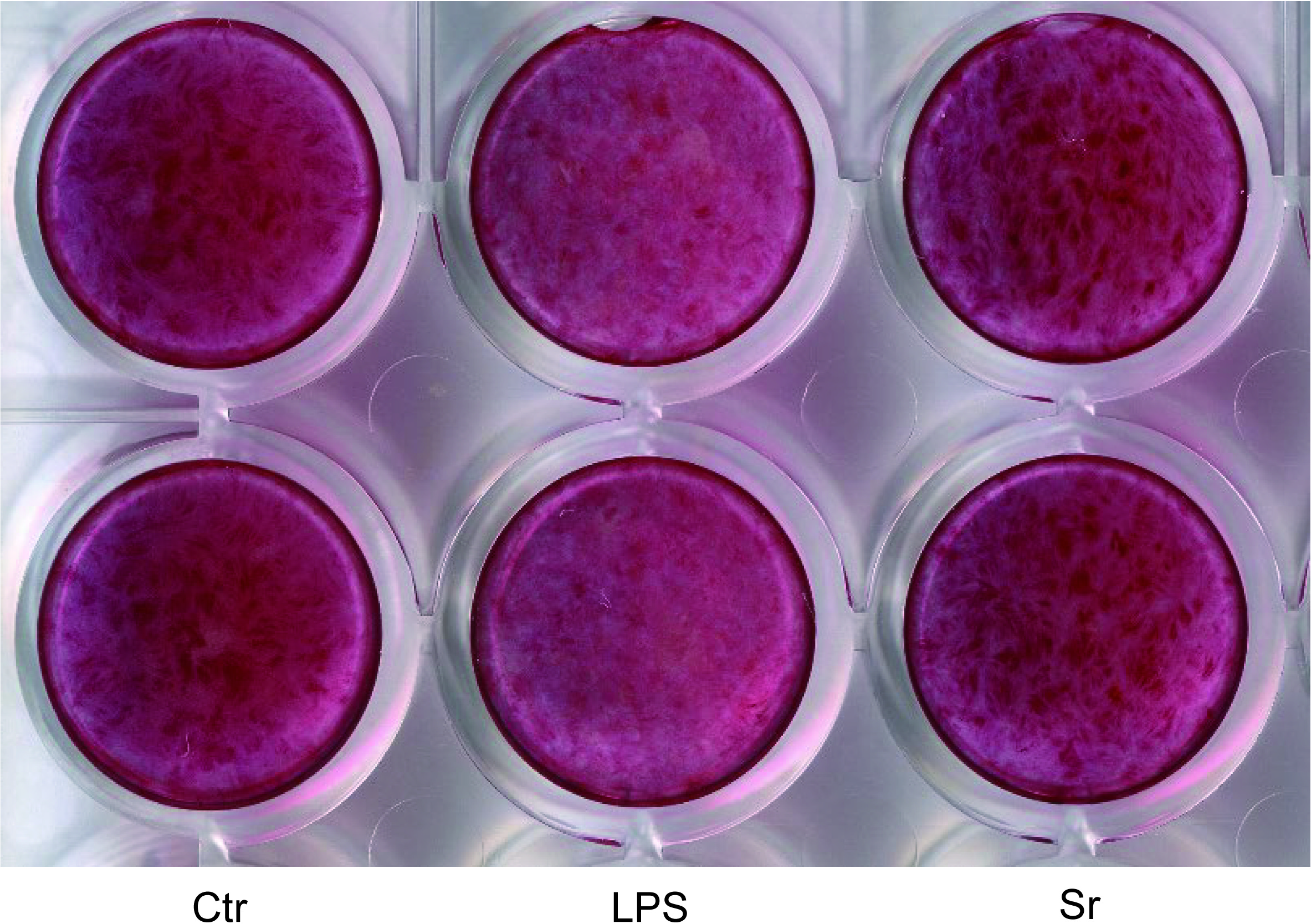
The lowest and highest degrees of staining were observed for the LPS and strontium groups, respectively.

Collagen production was highest in the control group and lowest in the LPS group (n = 6).
The lowest degree of staining was observed for the LPS group.
Collagen production was measured after 7 days of incubation. As shown in Fig. 5, significant differences were observed between all groups (P < 0.05, Tukey’s honestly significant difference test). Collagen production was the highest in the control group and the lowest in the LPS group. The collagen staining images are presented in Fig. 6.
It is said that bioactive dental material such as S-PRG filler eluate releases ions (Na+, F−, Al3+, BO33−, Sr2+, and SiO32−) [8], some of which promote hard tissue formation or remineralization [16]. S-PRG filler eluate has also been shown to promote the osteoblastic differentiation of human MSCs [17]. Human MSCs and MC3T3-E1 cells are commonly used as model systems to study osteogenic differentiation, and they undergo a similar mineralization process. MC3T3-E1 cells are non-transformed murine osteoblasts that can differentiate into premature and mature osteoblasts [18]. Osteoblastic differentiation is characterized by cell proliferation, differentiation, and mineralization. ALP activity is a widely used marker for early osteoblastic differentiation [19]. The aim of this study was to investigate which ions affect the osteogenic response of MC3T3-E1 cells in vitro. Strontium ions can activate osteoblast activity and inhibit osteoclast activity.
Boron, silicon, and strontium are considered to promote bone formation by inducing osteoblastic differentiation of undifferentiated mesenchymal cells [20]. Meanwhile, sodium fluoride promotes the proliferation of osteoblasts in a dose-dependent manner and aluminum ions at high concentrations contribute to cell death in cell proliferation experiments [21].
Aluminum ions at concentrations of 10-40 ppm were reported to significantly decrease the expression of osteogenic genes in cultured primary rat osteoblasts and suppress mineralization in a dose-dependent manner [22]. In another work, aluminum ions at concentrations of 2.8-28.4 ppm were found to significantly reduce the viability of cultured primary rat osteoblasts and suppress the expression of osteogenic marker genes [23]. These findings suggest that high concentrations of aluminum ions contribute to cell death in cell proliferation experiments [21]. Furthermore, 2 mM AlCl3 was reported to induce ultrastructural damage, oxidative stress, mitophagy, apoptosis, and cellular dysfunction in MC3T3-E1 cells [24].
S-PRG filler eluate diluted to contain 0.012-0.12 ppm of aluminum ions was reported to be compatible with human gingival fibroblasts without displaying cytotoxicity [21]. In addition, S-PRG filler eluate diluted to contain 0.013-0.025 ppm of aluminum ions did not affect osteoblastic differentiation in UE7T-13 human bone marrow-derived MSCs [17].
Boron promotes the expression of bone differentiation markers and is reported to have potential osteoinductive effects. Inhibition of cell proliferation was observed when human dental-pulp-derived stem cells (hDPSCs) were cultured in S-PRG filler eluate containing ≥1 mM boron [25]. However, media containing boron concentrations of 230, 247, and 280 μM but almost no other components, had no effect on the proliferation of hDPSCs after culturing for 2 days. The ALP activity in the presence of 247 μM boron was higher than that of the control although proliferation was significantly suppressed, and at 280 μM the ALP activity increased remarkably. These results suggest that the release of boron from S-PRG fillers could increase the ALP activity of hDPSCs.
Boron plays a crucial role in osteogenesis and bone maintenance and has important effects on the mineralization and morphogenesis of MC3T3-E1 cells. The addition of 0.1-10 ng/mL of boron was reported to promote calcification without affecting viability in MC3T3-E1 cells [26]. Furthermore, the addition of diluted S-PRG filler eluate to obtain a medium containing 1.31 ppm (121 μM) of boron promoted the calcification of UE7T-13 human bone-marrow-derived MSCs [17]. In addition, primary rat bone-marrow-derived MSCs cultured in the presence of boron concentrations ranging from 6 ng/mL to 6 μg/mL displayed significantly induced ALP activity after the 10th day [20]. These findings suggest that boron ions at concentrations of 0.0001-6 ppm may have the ability to induce osteogenic differentiation of pre-osteoblasts or MSCs, although high doses of boron ions (1 ppm) may cause cytotoxicity against undifferentiated MSCs [26, 20].
There have been no studies reporting that fluoride promoted bone formation. At the same time, fluoride may exert dose-dependent toxic effects [27]. Sodium fluoride at 50-100 μM (i.e., approximately 2.1-4.2 μg/mL) releases 1.0-1.9 μg/mL (ppm) of fluoride ions, which has been reported to enhance cell proliferation and promote osteogenic differentiation of human dental pulp cells [28]. Fluoride ion concentrations of 10-50 ppm inhibit human dental pulp cell proliferation, and a fluoride ion concentration of 50 ppm was reported to be a lethal dose for human pulp cells [29], such that high concentrations of fluoride ions may cause cell death in proliferation experiments [21]. Fluoride ions are considered a multifunctional growth factor with diverse effects on the proliferation and differentiation of osteoblasts. For example, sodium fluoride promotes the osteoblastic differentiation of MC3T3-E1 cells and enhances ALP activity and matrix mineralization in a dose-dependent (50 or 500 μM) manner. However, at high doses (5 mM), sodium fluoride led to apoptosis and autophagy and affected the proliferation and survival of MC3T3-E1 cells [30].
Like calcium, strontium occurs naturally in bone and teeth, albeit in trace amounts. Strontium ions have been reported to promote bone formation, where a low concentration was most effective in inducing bone formation [31]. Previous studies have also reported that strontium was able to positively modulate osteogenic differentiation at concentrations ranging from 1 to 10 mM in osteoblast cells [32]. The effects of strontium on cell proliferation have been investigated. The addition of 1.5 mM of SrCl2 promoted chondrocyte differentiation of dedifferentiated fat cells, while 3 mM of SrCl2 promoted the differentiation of MC3T3-E1 cells [33].
Previous studies demonstrated that strontium at 12.5 μM (i.e., approximately 1.1 μg/mL) did not affect the proliferation of the murine cementoblast cell line OCCM-30 but significantly promoted the expression of osteogenic genes in these cells. High concentrations of strontium ranging from 25 to 200 μM (i.e., approximately 2.2-17.6 μg/mL) significantly decreased the viability of OCCM-30 cells, whereas a concentration of 12.5 μM (1.1 ppm) had no effect. S-PRG filler eluate which was diluted to obtain final strontium ion concentrations of 0.79 or 1.6 μg/mL, was found to be osteoinductive with the ability to promote osteoblastic differentiation in UE7T-13 cells [17]. S-PRG filler eluate diluted to afford final strontium ion concentrations of 0.7928-7.928 ppm was not cytotoxic to human gingival fibroblasts [21].
With respect to MC3T3-E1 cells, 1 mM of strontium ions was found to stimulate proliferation and increase the mRNA levels of osteocalcin and bone morphogenetic protein 2 [34]. In accordance with the previous results, a strontium ion concentration of 1 mM also led to osteoblastic differentiation in this study. The concentration of strontium in S-PRG filler [14] is close to this amount. An LDH assay was used to evaluate the occurrence of cytotoxicity. The results indicated that strontium ions were not cytotoxic to MC3T3-E1 cells and did not adversely affect osteoblastic differentiation at the concentrations used. The boron ions alone could be cytotoxic to the cells, but the combination of ions together may interact in a manner that suppresses this effect.
In conclusion, media containing 1 mM strontium ions promoted osteoblastic differentiation in MC3T3-E1 cells without displaying cytotoxicity. Future research is warranted to determine the minimum concentration and the culturing time for each ion released by S-PRG fillers.
NT: conceptualization, investigation, methodology, data curation, formal analysis, visualization, and writing. YT: conceptualization, methodology, formal analysis, writing, review, editing, and supervision. TG: investigation. MI: formal analysis, writing, review, editing, and supervision. YS: conceptualization, methodology, formal analysis, writing, review, editing, and supervision. All authors read and approved the final version of the manuscript.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
The data supporting the findings of this study are available from the corresponding author, NT, upon reasonable request.
This research was supported by a Grant-in-Aid for Scientific Research (No. 20KK0209) from the Japan Society for the Promotion of Science.
2) YT: tamu.hpha@tmd.ac.jp, https://orcid.org/0000-0002-4287-5927
1) TG: tadamu.g.0131@gmail.com, https://orcid.org/0000-0003-4933-3394
3) MI: ikeda.csoe@tmd.ac.jp, https://orcid.org/0000-0003-2214-4980
1) YS: shimada.ope@tmd.ac.jp, https://orcid.org/0000-0002-0751-4819
The authors thank Dr. Mahmoud Sayed at the Oral Health Science Center, Tokyo Dental College for assistance in figure preparation.