2023 年 20 巻 1 号 論文ID: e200014
2023 年 20 巻 1 号 論文ID: e200014
Spider silk is considered a promising next-generation biomaterial due to its exceptional toughness, coupled with its renewability and biodegradability. Contrary to the conventional view that spider silk is mainly composed of two types of silk proteins (spidroins), MaSp1 and MaSp2, multi-omics strategies are increasingly revealing that the inclusion of complex components confers the higher mechanical properties to the material. In this review, we focus on several recent findings that report essential components and mechanisms that are necessary to reproduce the properties of natural spider silk. First, we discuss the discovery of MaSp3, a newly identified spidroin that is a major component in the composition of spider silk, in addition to the previously understood MaSp1 and MaSp2. Moreover, the role of the Spider-silk Constituting Element (SpiCE), which is present in trace amounts but has been found to significantly increase the tensile strength of artificial spider silk, is explored. We also delve into the process of spidroin fibril formation through liquid-liquid phase separation (LLPS) that forms the hierarchical structure of spider silk. In addition, we review the correlation between amino acid sequences and mechanical properties such as toughness and supercontraction, as revealed by an analysis of 1,000 spiders. In conclusion, these recent findings contribute to the comprehensive understanding of the mechanisms that give spider silk its high mechanical properties and help to improve artificial spider silk production.
Plastic products, which are widely used today, have a significant environmental burden due to carbon emissions and the problem of microplastics. As a result, there has been increasing interest in biodegradable biomaterials that do not require petroleum as a raw material. Among these materials, spider silk is considered the toughest material combining strength and elasticity. Understanding of the primary sequence design, components and their stoichiometry, as well as the molecular spinning process are thus essential to achieve artificial production in an industrial scale.
Silk has been valued as a luxurious and versatile material for centuries, and the Silk Road was a major trade route for silk fabrics from the 2nd century BC to the 15th century. Silkworms produce silk that is not only used in clothing, but also has a variety of applications in fields such as medicine as a biologically-derived material. In the late 20th century, the oil industry saw significant growth, leading to the widespread adoption of synthetic fibers like nylon and polyester as substitutes for natural fabrics. However, synthetic fibers have environmental drawbacks, such as the release of carbon and microplastics, as they are made from oil are not biodegradable [1,2]. In an era where sustainable development goals (SDGs) are being set globally and a new industrial revolution driven by biotechnology is sought, protein materials designed using protein engineering have garnered renewed interest as the next generation of high-performance materials, that are highly functional, renewable, and biodegradable [3]. Many arthropods especially in the order Lepidoptera utilize silk, with a tensile strength of about 0.5 GPa in Bombycidae (silkworms) and 0.7 GPa in Psychidae (bagworms). Silk from orb weaving spiders often surpasses arthropod silk in strength, with a tensile strength of more than 1 GPa and a degree of extensibility (30%) comparable to that of nylon. This makes spider silk one of the toughest of all natural materials [4–6]. However, breeding spiders is challenging due to their carnivorous nature and tendency to engage in cannibalism, making the mass production of natural fibers difficult. In recent years, there have been promising developments in the effort to industrialize artificial spider silk, including the synthesis of spider silk through microorganisms [7] and synthesis of polypeptides that mimic its molecular structures [8]. While much is still unknown about the relationship between the arrangement and properties of spider silk, reproducing the exceptional properties of natural spider silk requires a deeper understanding of the molecular-level structural design principles and mechanisms of protein materials [9–12]. Recent advances in high-throughput molecular biology, including genomics, transcriptomics, and proteomics collectively referred to as multi-omics have made it possible to conduct analyses in this regard. This review article is an extended version of the Japanese article [13].
Approximately 50,000 species of spiders have been currently described, all of which are carnivores that utilize silks in some capacity. Although spiders are often associated with orb webs, this trait is actually only found in less than half of all species. Spider silks are used for a variety of purposes, including nesting, prey capture, migration, reproduction, egg protection, and signal sensing, and the exceptional toughness of dragline silk presumably evolved through evolutionary optimization as a result of this diversity. Accordingly, rather than using a single type of silk for multiple purposes, spiders produce and use different types of silk for specific purposes. For example, orb-weaving spiders use up to seven different types of silk with different properties, including strong silks for web frames (Major ampullate spidroin, MaSp), scaffold silks for auxiliary spiral of the orb web (Minor ampullate Spidroin, MiSp), highly elastic silk for prey trapping (Flagelliform spidroin, FlSp) coupled with adhesive silks (Aggregate glue, Agg), and etc [14]. The overview of spider silk structure and its spinning process is depicted in Fig. 1. Spider silks are produced in specific silk glands, and they transition from initial liquid state into solid fiber state in these silk-producing organs as silk proteins pass through the silk gland. Fibrillation occur under the influence of the ion gradient and shear stress. Spider silk is composed of skin and core bundle of nanofibrils, with the amino acids of the nanofibrils forming a secondary structure that repeatedly alternates between crystalline domains (beta sheets) and amorphous domains (random coils). While the length and amino acid composition of silk genes vary among species and types of spidroins, phylogenetic analysis of silk genes has suggested that all diversified genes are monophyletic [15]. In other words, over the course of 380 million years of evolution, spiders have gradually changed, optimized, and diversified genes with shared ancester into specified silks resulting in highly strong or elastic silks. Therefore, it is thought that by collecting and tracing the evolution of the various existing systems of spider silk genes and their properties, it is possible to determine which sequence characteristics, i.e., mutations, contribute to which properties. Spidroins have gene lengths around or exceeding 10 kbp which is about ten times longer than that of typical genes [16–18], and is further composed largely of repeat sequences, making it extremely difficult to determine its full-length sequence. In recent years, the advent of nanopore sequencers has made it possible to perform long-read analysis, realizing full-length sequencing of repetitive sequences [19]. Hybrid error-correction strategy was proposed to combine long-read sequencing to capture the full-length of the repetitive gene, and short read sequencing for accurate error-corrections by repeat-unit assembly [20].

(A) Schematic illustration of the spinning process and the hierarchical structure of spider silk. The major ampullate gland contains liquid-phase proteins that constitute the silk, including spidroins, cysteine-rich proteins (CRP) and spider silk constituting elements (SpiCE). The final solid-state silk released is composed of a surface coating skin and the core bundle of nanofibrils. (B) Spidroin is composed of repeating domains consisting of crystalline and amorphous regions and N/C-terminal domains that play roles in the fibrillization process. (C) The repeat sequences and exonic regions of MaSp1, MiSp, and AgSp in Araneus ventricosus. The composition, length and number of repeated amino acids vary depending on the type of spidroin.
It has been widely believed that MaSp1 and MaSp2, two types of Spidroin paralogs, are the proteins that make up the toughest spider dragline silk [21]. However, this is mostly due to the lack of comprehensive reference sequence set because of the difficulty in determining the full length of the spidroin gene sequences. In order to address this, Kono et al. sequenced and assembled the genome of a common orb weaving spider Araneus ventricosus especially curating for the complete set of full-length spidroins with nanopore long reads [22]. The genome of A. ventricosus harbored MaSp3, whose partial fragment was previously reported by Collin et al. with a characteristic DGGR motif in the repetitive regions [23]. While the pralogous nature of MaSp3 with other MaSp families of spidroins was confirmed by phylogenetic analysis of the N-terminal sequences, it also formed a distinct clade, hence worthy of its assignment as a novel MaSp paralog. Furthermore, MaSp3 is equally highly abundant as MaSp1 and MaSp2, confirmed by gland-specific transcriptomics as well as the silk proteomics, using the curated spidroin set as the reference. Such abundance was confirmed in the following study of the comprehensive spidroin catalogue of Nephilinae spiders (Fig. 2) [24]. By using the full-length spidroin sequences, Kono et al. also identified wide MaSp3 conservation within orb weaving spiders in Araneidae through comparative analysis of transcriptomes [22]. Then, is the combination of MaSp1,2 and 3 sufficient to explain the exceptional properties of spider dragline silk? Previous proteomic studies in fact suggested the presence of non-spidroin components such as cysteine-rich protein (CRP) and cysteine-rich secreted protein (CRISP3) [25–30]. Quantitative protemics using the curated reference data of Trichonephila clavata and A. ventricosus identified the consistent and reproducible inclusion of several non-spidroin proteins. Through phylogenetic profiling and computational prediction of the effects of these candidates on the toughness of the silk based on a multivariate analysis of a series of mechanical measurement coupled with proteomics with spiders sampled under various nutritional conditions, the most plausible candidates were named Spider-silk Constituting Element (SpiCE). While SpiCEs only comprise about 1–5% w/w of the dragline silk, its evolutionary co-conservation with MaSp3 strongly suggests its involvement in the physical properties of Araneidae silk [22].

Multi-omics analysis combining high-resolution genomic analysis including full-length spider silk gene sequences, transcriptomic analysis of silk glands, and proteomic analysis of dragline silks identified the presence of a novel Spidroin (MaSp3) and a small molecular weight protein, SpiCE. Reprinted from Ref. 24 Copyright (2021) National Academy of Sciences.
In order to characterize the effects of SpiCE on the mechanical properties of spider silk, Kono et al. then reconstituted SpiCE in vitro [24]. Recombinant SpiCE-NMa1 and MaSp2 from T. clavata were synthesized using E. coli, and composite films were prepared by adding 1%, 3%, or 5% (w/w) SpiCE-NMa1 to MaSp2 silk solution, and their physical properties were measured. As a result, the MaSp2-SpiCE mixed films consistently showed more than twice the tensile strength at 1% or more (Fig. 3A). While no strength changes were observed when creating artificial silk with a MaSp2-SpiCE mixture, a characteristic yield point typically seen with natural spider silks, was observed, which was not seen with MaSp2 alone. Yield point is a point in stress-strain curve indicating the limit of reversible elastic behavior, and the beginning of elasto-plastic behavior (Fig. 3B). As its name suggests, even in small quantities, SpiCE significantly improves the structure and mechanical properties of spider silk. The molecular mechanisms by which SpiCE affects physical properties are yet to be understood, but it was speculated to facilitate interactions between the multiple MaSp paralog proteins.

(A) Artificial spider silk film made with SpiCE and MaSp2, where the addition of 1% SpiCE (w/w) more than doubles the tensile strength (right graph). (B) Stress-strain curve of artificial spider silk produced by combining SpiCE and MaSp2, where the addition of SpiCE (right) causes the characteristic yield point of the spider silk. Modified from Ref. 24 Copyright (2021) National Academy of Sciences.
Synthetic films of MaSp1, 2, and 3 as well as their combinations were produced and tested likewise SpiCE, and WAXS (wide-angle X-ray scattering) analysis suggested the primary contribution of MaSp1 on the strength due to crystalline regions, and MaSp2 on the strain due to amorphous regions, confirming previous structural analyses [31,32]. MaSp3 showed structural features in between the two paralogs, and thus can be considered to contribute to the toughness of silk. In any case, tough spider silk requires all three of these components as well as SpiCEs to be fully reconstituted, with proper hierarchically orientated structure. It should also be noted that there seem to be multiple SpiCEs present within the dragline, and presumably specific SpiCEs exist in each type of spider silk. For example, the dragline silk of T. clavata contains at least four SpiCEs, and SpiCE-NMa2 and SpiCE-NMa4 have high cysteine content somewhat resembling CRPs, at least in terms of their amino acid composition. Likewise, Darwin’s bark spider (Caerostris darwini), known for spinning the toughest silk among all spiders, has been found to possess a MaSp paralog named MaSp4, which is believed to have originated from MaSp2 and utilizes a unique beta-sheet motif instead of a poly-Alanine [33]. Therefore, exploration of different MaSp paralogs as well as SpiCEs as natural enhancing additives could be an interesting topic for future researches.
Nearly 90% of spidroin is comprised of the repetitive sequences, which is the key component in shaping the extraordinary mechanical properties of the spider silk; however, the conserved N/C-termini are also essential during the self-assembly of spidroins to form the hierarchically organized silk thread. In the spider body, spidroin exists as a highly concentrated protein solution (~50% protein) within the silk gland [34–36], and the protein structure gradually change from an unfolded structure to a nanocrystalline β-sheet structure under the influence of an ionic gradient from pH 8 to 5 as the protein dope move along the gland towards the spinning duct [37–39]. The two terminal domains dimerize in the process, and several essential amino acid residues associated with dimerization, such as the conserved acidic residues in the N-terminus (D40, E79, E84, and E119) are reported [40,41]. However, it had been difficult to artificially mimic this hierarchical structure of the spinning process until recently, Malay et al. successfully reproduced the natural spinning process in vitro and revealed the mechanism of hierarchical structure formation [42]. Using synthetic MaSp2, Malay et al. showed that this protein solution undergoes liquid-liquid phase separation (LLPS) at neutral pH in the presence of phosphates, resulting in the formation of silk protein droplets. Furthermore, by lowering the pH after LLPS formation, the droplet became fibrous. Finally, when a shear stress was applied to the fibrillated silk proteins, the oriented fibers bundled together to reproduce the characteristic hierarchical fiber structure. (Fig. 4).

(A) The schematic figure of the conformational changes of spidroins as they pass through the Major Ampullate Gland, influenced by the pH gradient (from neutral to acidic) and ion exchange, causing the CTD and NTD to dimerize. (B) Spider silk proteins undergo liquid-liquid phase separation under phosphate conditions at neutral pH, and further fibrillation occurs when the pH shifts to acidic conditions. Shear stress in this fibrillated state leads to the formation of a hierarchical fiber structure. Scale bar is 10 μm, Reproduced and modified from Ref. 42 under Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
To investigate the role of the N/C-terminal domains in LLPS and fiber formation, Malay et al. tested MaSp2 constructs lacking all possible combinations of N/C terminal and repetitive domains for the effect of pH gradient. This series of experiments clearly demonstrated that the C-terminal domain plays a central role in LLPS, and that the N-terminal domain is essential for subsequent fibril network assembly. Therefore, silk spinning under conditions inducing LLPS would be critical in artificial silk construction with self-assembly, and a homogeneous and robust intermolecular interactions of multiple MaSp paralogs would be essential in reproducing the physical properties of natural spider silk.
As our understanding of the elements that make up spider silk as well as the process of spinning it grows, there is still much to learn about the influence of spidroin sequences on mechanical properties. For instance, while dragline silk exhibits exceptional mechanical strength, its tendency to shrink up to 60% in length when wet (a phenomenon known as supercontraction) makes it less desirable for industrial use [43,44]. By understanding the unique amino acid motifs of each spidroin and their correlation with specific properties of silk, we may be able to design and modify the primary sequence of artificial spider silk to suit our needs [45]. With this goal in mind, Arakawa et al. collected over 1,000 spiders and analyzed the physical properties of their dragline silk, as well as the comprehensive transcriptome sequence of the spiders, in order to gain insight into the relationship between spidroin sequence and mechanical properties [46]. By sampling 1,774 spiders belonging to 1098 species across 76 families in 441 genera from four continents, they catalogued a total of 11,155 spidroin genes through the curation of transcriptome assemblies. This included aciniform Spidroin (AcSp), pyriform spidroin (PySp), and cribellar spidroin (CrSp) from nine previously unknown families [47], in addition to previously unidentified spidroin sequences from the Tetragnathidae and Linyphiidae, two major subfamilies of Araneidae. Spidroins were the most diverse in Araneidae, and spidroins related to flag and aggregate silk (Flag and Agg) were conserved only within the superfamily Araneoidea.
Together with the spidroin sequence data, dragline silks were collected and evaluated using 12 indicator parameters: mechanical properties (toughness, Young’s modulus, tensile strength, and strain at break), morphological and structural properties (fiber diameter, birefringence, and degree of crystallinity based on wide-angle x-ray scattering (WAXS) analysis), thermal degradation profiles (onset temperature for 1, 5, and 10% weight loss), and hydration properties (fiber water content and degree of maximum supercontraction). The data obtained from these tests may vary significantly due to external factors such as humidity and silking speed, making it difficult to compare results reported in different literature (Fig. 5A). However, the data presented by Arakawa et al. allows for comprehensive comparison as it was all obtained under a single standardized protocol. The distribution of mechanical properties yielded values of up to 0.45 GJ/m3 for toughness, up to 60% for strain at break, and up to 3 GPa for tensile strength, and the wide range of mechanical properties among spiders makes it a promising subject for studying the relationship between amino acid sequences and lineage. All data, including spidroin sequences and mechanical properties, are publicly available from the Spider Silkome Database (https://spider-silkome.org) (Fig. 5C). It is one of the largest collections to date linking genotype to extended phenotype for a specific type of protein biomaterial.
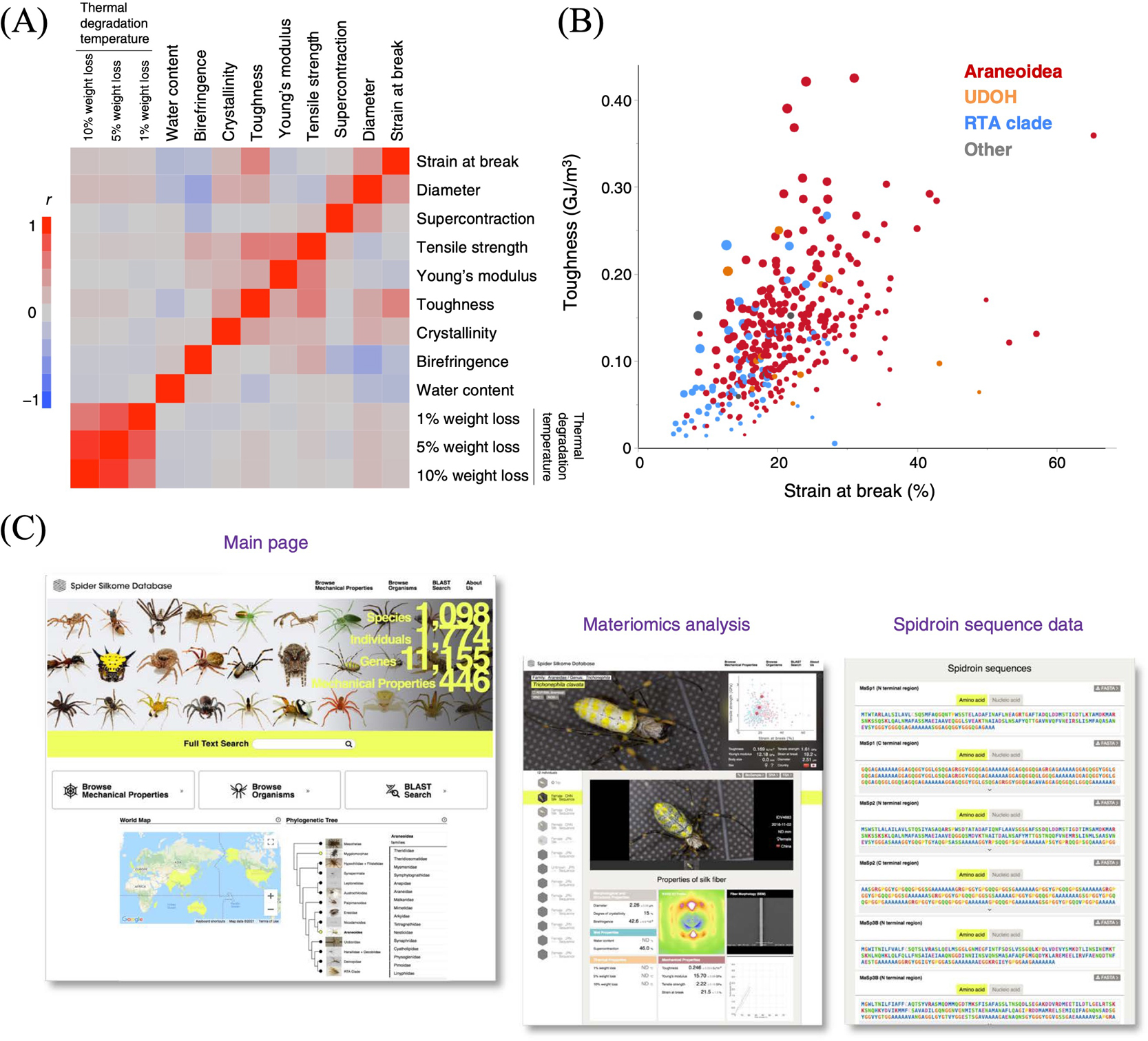
Overview of the physical properties of 446 spider silk samples. (A) Pearson correlation heatmap of the physical properties of dragline silk fibers measured in Arakawa et al. [46]. Toughness is not only correlated with tensile strength and strain at break but also correlated with Young’s modulus. Supercontraction is correlated with strain at break. (B) Scatter plot of toughness versus strain at break (with spot size proportional to tensile strength). The collected samples represent an almost continuous spectrum of toughness from <0.01 to >0.40 GJ/m3. Spots are colored according to broad phylogenetic grouping: Araneoidea (red), RTA clade (light blue). (C) Screenshots of the Spider Silkome Database (https://spider-silkome.org). Reprinted from Ref. 46 under Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
The main components of dragline silk, MaSp1, MaSp2, and MaSp3, can be divided into 7, 4, and 2 subtypes, respectively. Among these, one subtype of MaSp3 showed the highest average difference in silk toughness, at +0.041 GJ/m3. This value corresponds to approximately a 32% increase from the overall average value of 0.127 GJ/m3, indicating that MaSp3 is an essential element for tough dragline silk. Many subtypes of MaSp2 showed supercontraction, with some subtypes showing up to a 15.7% increase. However, some subtypes of MaSp2 also suppress supercontraction, with longer polyalanine regions in the crystalline domain than normal subtypes. When looking at the average amorphous/beta sheet region length ratio of all repeats in MaSp1 and MaSp2 spidroins, MaSp1 was 0.526 and MaSp2 was 0.394. Previous research [48] has suggested that the relaxation of orientation in the amorphous region of spidroins contributes to supercontraction, and this trend has also been confirmed in the comprehensive data set of Arakawa et al. [46].
Next, Arakawa et al. extracted amino acid motifs correlated with the measured properties. As a result, supercontraction showed a strong negative correlation with the proportion of polyalanine sequences, as well as with short (1–4 amino acids) motifs corresponding to amorphous regions such as G, GG, and AGQG (Fig. 6). Likewise, strain at break was negatively correlated with polyalanine headed by Ser in MaSp2, and was positively correlated with MaSp1/2 amorphous regions containing Pro. Tensile strength was negatively affected by the presence of Ala in the amorphous region of MaSp1, and Pro in the amorphous region of MaSp2, and in turn positively affected by the presence of Ser in the amorphous region of MaSp1. GYGQGG motif in MaSp1 correlated most strongly with both tensile strength and fracture strain, and consequently also with toughness. Moreover, Pro in the amorphous region of MaSp2 was negatively correlated with toughness, as were the SY and SV motifs of MaSp1, and the presence of GGS after the polyalanine region of MaSp1 was positively correlated with toughness. Thus, using engineered constructs to confirm the contribution of motifs to properties is a promising direction for fully understanding the primary sequence design that contributes to the extraordinary mechanical properties of spider silk.

Scatterplot of physical properties (toughness or supercontraction) as a function of the average motif abundance per repeat of certain amino acid motifs. AGQG motif in MaSp1 is positively correlated with supercontraction, AAAAAAAA motif of MaSp2 is negatively correlated, and YGQGG motif in MaSp1 is positively correlated with toughness. Reprinted and modified from Ref. 46 under Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
In summary, these data again indicate that MaSp1 is related to strength, MaSp2 to elasticity, and MaSp3 to silk properties for exceptional toughness. These comprehensive analyses have demonstrated not only the feasibility of designing primary sequences that preserve the material’s toughness while avoiding supercontraction. The vast amount of transcriptome data available are useful for other areas of biology, such as advancing arachnid and arthropod phylogeny [49,50].
Spider silk is a high-performance next-generation biomaterial with exceptional physical properties and biodegradability, making it a sustainable alternative to synthetic fibers, and a potential contributor to the Sustainable Development Goals (SDGs). Molecular mechanisms realizing the extraordinary properties of spider silk is beginning to be uncovered, with the advent of omics technologies. Firstly, the full list and stoichiometry of the components of dragline silk was revealed by long-read sequencing and quantitative proteomics, and a non-spidroin component SpiCE was identified as a minor but essential component for silk strength, which doubles the tensile strength of artificial spider silk in vitro. Secondly, the self-assembly process of spidroins involves LLPS, and the N/C terminal domains are essential for this purpose. Spider silk assembled via LLPS realizes the hierarchically bundled structure. Thirdly, through the study of 1000 spiders from all over the world, relationships between spidroin sequences and mechanical properties have been identified, and this data has been compiled in the publicly available Spider Silkome Database. Together, these recent findings lay the foundation for the industrial-scale application of spider silk.
The authors declare no conflict of interest associated with this manuscript.
The manuscript was written through contributions of both authors and both authors have given approval to the final version of the manuscript.
The evidence data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by a grant from the ImPACT Program of the Council for Science, Technology and Innovation (Cabinet Office, Government of Japan), MEXT Program: Data Creation and Utilization-Type Material Research and Development Project Grant Number JPMXP1122714694, KAKENHI Grant Number FX221015E2, and by research funds from the Yamagata Prefectural Government and Tsuruoka City, Japan.