2013 Volume 36 Issue 12 Pages 1921-1927
2013 Volume 36 Issue 12 Pages 1921-1927
Triple-negative breast cancer (TNBC) has a poor prognosis compared to other subtypes, and effective treatment options are limited to cytotoxic agents, including microtubule-targeting agents, due to the lack of molecular targets. Here, we examined the combined effect of sepantronium bromide (YM155) and microtubule-targeting agents in TNBC models. The combination of YM155 with docetaxel showed synergistic antiproliferative and caspase 3/7-inducing effects in MRK-nu-1 and MDA-MB-453 human TNBC cell lines in vitro. YM155 also synergistically enhanced the efficacies of other microtubule-targeting agents, including paclitaxel and vinorelbine, which induced accumulation of survivin at the G2/M phase, whereas it did not affect the efficacy of doxorubicin. Combination treatment with YM155 and microtubule-targeting agents decreased the accumulation of survivin at the G2/M phase and induced greater apoptosis than either single agent alone. Further, combination treatment with YM155 and docetaxel also had a synergistic antitumor effect, achieving complete regression without exacerbation of body weight loss in all mice, in a MRK-nu-1 human TNBC xenograft model. These results suggest that survivin inhibition synergistically sensitize human TNBC cells to microtubule-targeting agents.
Breast cancer is the second leading cause of cancer death among women in the United States.1) Triple-negative breast cancer (TNBC) is a clinical phenotype characterized by a lack of estrogen receptor (ER) and progesterone receptor (PR) expression as well as an absence of human epidermal growth factor-2 (HER-2) overexpression. Retrospective studies show that TNBC represents approximately 15% of total breast cancers and suggest that this cancer type is aggressive, with rapid tumor growth, a high incidence of metastasis, an increased possibility of distant recurrence, and a higher mortality rate than other breast cancers.2–4) Unlike patients with an ER/PR-positive or HER-2-overexpressing subtype, this lack of molecular targets limits systemic treatment options for patients with TNBC to cytotoxic chemotherapy. Among them, microtubule-targeting agents, such as taxanes and vinca alkaloids are one of the most common class of chemotherapeutic drugs for the treatment of TNBC.5,6) Despite the sensitivity of TNBC to chemotherapy, however, prognosis remains poor. The median time to death was shorter in patients with TNBC than in those with other subtypes.7) Moreover, patients with TNBC who had residual disease after neoadjuvant chemotherapy had a particularly poor outcome.8) This poor prognosis and lack of therapeutic agents has underscored the demand for novel therapeutic options and strategies.
Survivin, a member of the inhibitor of apoptosis (IAP) family of proteins, has been implicated in both cell survival and regulation of mitosis.9) Overexpression of survivin is observed in various types of cancer, including breast cancer.10,11) Inhibition of survivin using ribozyme and a dominant-negative mutant induced spontaneous apoptosis and decreased the growth rate of breast cancer cells.12,13) Moreover, survivin small interfering RNA (siRNA) and a dominant-negative mutant of survivin enhanced the antitumor activity of taxanes in several types of cancer.13–15) Taken together, these findings suggest that survivin is an attractive target for the treatment of breast cancers and that inhibition of survivin might be an effective way of enhancing the antitumor activities of microtubule-targeting agents.
Sepantronium bromide (YM155), a survivin suppressant, shows nanomolar antiproliferative activity against a broad range of human cancer cell lines and induces tumor regression in non-small cell lung cancer (NSCLC), melanoma, bladder, aggressive non-Hodgkin lymphoma, and breast cancer xenograft models.16,17) In clinical settings, YM155 has shown modest clinical activity in patients with advanced refractory NSCLC18) and unresectable melanoma,19) and further development of YM155 in combination with other anticancer agents was suggested. In a large cell panel screening, breast cancer ranked third among YM155-sensitive tumor types.16) Further, YM155 reduced spontaneous metastases and significantly prolonged the survival of animals bearing established metastatic tumors derived from a human TNBC cell line.20) Consistent with the findings from survivin siRNA and dominant-negative mutant studies, YM155 enhanced the efficacy of docetaxel in NSCLC21) and melanoma cells.22) Therefore we hypothesized that survivin suppression by YM155 would enhance the antitumor activities of microtubule-targeting agents in TNBC. Here, we examined the combined effect of YM155 and microtubule-targeting agents on TNBC and investigated possible underlying mechanisms of action.
Human breast cancer cell lines MRK-nu-1 (JCRB0628) and MDA-MB-453 (HTB-131) were obtained from the Japanese Collection of Research Bioresources (Osaka, Japan) and the American Type Culture Collection (Manassas, VA, U.S.A.), respectively. Cells were cultured in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere.
ReagentsYM155 was synthesized by Astellas Pharma Inc. (Tokyo, Japan). Paclitaxel was purchased from LC Laboratories (Woburn, MA, U.S.A.). Docetaxel, doxorubicin, and vinorelbine were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). For in vitro experiments, test compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted with culture medium (DMSO final concentration in culture medium: 0.1% [v/v]). For in vivo studies, the dose of YM155 was expressed as that of the cationic moiety of the drug. Docetaxel (Taxotere® injection) was purchased from Sanofi Aventis Co., Ltd. (Bridgewater, NJ, U.S.A.). All drugs were dissolved and diluted in saline just before administration.
In Vitro Assay for Cell Viability and Caspase 3/7 ActivityCell viability and caspase 3/7 activity were determined using a CellTiter-Glo® Luminescent Cell Viability Assay and a Caspase-Glo3/7® assay (Promega, Madison, WI, U.S.A.), respectively. The luminescence of each sample was measured in a plate-reading luminometer (EnVision®; Perkin-Elmer, Waltham, MA, U.S.A.). Luminescent signals for drug-treated cells were normalized to that of control cells.
Analysis for SynergyThe Bliss additivism model23,24) was used to classify the effect of combining two agents as additive, synergistic, or antagonistic. A theoretical curve was calculated for combined inhibition using the equation Ebliss: bliss index=EA+EB−EA×EB, where EA and EB are the fractional inhibitions obtained by drug A alone and drug B alone at specific concentrations. The combined effect of the two drugs was judged as follows, EA+B>Ebliss: synergistic, EA+B=Ebliss: additive, and EA+B<Ebliss: antagonistic.
Measurement of YM155 Concentrations in VitroMRK-nu-1 cells were seeded at 6×105 cells/mL in 6-well plates. After overnight incubation, cells were treated with docetaxel at 100 nmol/L for 3 h followed by YM155 at 30 nmol/L for 4 h. Cells were harvested, washed with phosphate buffered saline (PBS) and extracted with 0.1% formic acid. YM155 concentration was measured by LC/MS/MS using a Prominence 2000 HPLC (Shimadzu, Kyoto, Japan) coupled to an API-3000 MS/MS system (AB SCIEX, Foster City, CA, U.S.A.).
Western Blot AnalysisProtein was extracted using lysis buffer (RIPA Buffer [Thermo Fisher Scientific, Waltham, MA, U.S.A.], 1×Halt Phosphatase Inhibitor Cocktail [Thermo Fisher Scientific] and protease inhibitor cocktail [Sigma-Aldrich]). Protein concentration of the lysates was determined by the BCA Protein Assay Reagent Kit (Thermo Fisher Scientific). Equal amounts of total protein were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking at room temperature with Blocking One (Nacalai Tesque, Kyoto, Japan), each membrane was incubated overnight at 4°C with the following primary antibodies: anti-survivin (R&D Systems, Minneapolis, MN, U.S.A.), anti-phospho histone H3 (ser 10), anti-cleaved poly(ADP) ribose polymerase (PARP) (Cell Signaling Technology Inc., Danvers, MA, U.S.A.), or anti-β-actin (Sigma-Aldrich). After washing with TBS-T, membranes were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Proteins of interest were visualized by enhanced chemiluminescence using ECL-Plus (GE Healthcare, Fairfield, CT, U.S.A.).
Cell Cycle AnalysisCell cycle distribution was determined using a Guava PCA microcytometer (Guava Technologies, Hayward, CA, U.S.A.). Cells were fixed with ice-cold 70% ethanol and incubated at 4°C. Ethanol-fixed cells were washed with PBS and resuspended in Guava Cell Cycle Reagent (Guava Technologies). Data were collected and analyzed using CytoSoft software (Guava Technologies).
MRK-nu-1 Mice Xenograft ModelAll animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Astellas Pharma, Inc. The Tsukuba Research Center of Astellas Pharma, Inc. has been awarded Accreditation Status by AAALAC International. MRK-nu-1 cells were mixed with Matrigel® (Becton Dickinson Co., Franklin Lakes, NJ, U.S.A.), and 3×106 cells were subcutaneously (s.c.) injected into the flanks of female nude mice (CAnN.Cg-Foxn1nu/CrlCrlj (nu/nu)). After tumors of about 100 mm3 were established, mice were randomly assigned to groups based on tumor volume. The first day of administration was designated as day 0, and observation was continued until day 22. YM155 was administered at 2 mg/kg/d for 7 d by continuous s.c. infusion using a micro-osmotic pump (Alzet® model 1007D; Durect, Cupertino, CA, U.S.A.). Docetaxel was administered at 20 mg/kg intravenously (i.v.) once on day 0. Body weight and tumor diameter were measured twice a week, and tumor volume was determined (length×width2×0.5). Complete regression (CR) was defined as tumor regression to below the limit of palpation. Percent tumor regression was calculated using the following formula: 100×[1−(mean tumor volume of each group on day 22)/(mean tumor volume of each group on day 0)].
Statistical AnalysisValues were expressed as means±standard deviation (S.D.) or standard error (S.E.) and analyzed by Student’s t-test. Data analysis was performed using SAS software (SAS Institute, Cary, NC, U.S.A.), and p<0.05 was considered statistically significant.
We first evaluated the in vitro combined effect of YM155 and microtubule-targeting agents against TNBC cell lines. The combination of YM155 with docetaxel induced a greater decrease in cell viability than either agent alone (Fig. 1A). The percentages of cell growth inhibition induced by YM155 in combination with docetaxel were greater than the bliss index (Fig. 1B). Further, caspase 3/7 activity in cells treated with a combination of YM155 and docetaxel was greater than the sum of the activities in cells treated with each single agent alone (Fig. 1C). The combination of YM155 and paclitaxel or vinorelbine also showed greater cell growth inhibition than that of either agent alone (Fig. 2A). The percentages of cell growth inhibition induced by YM155 in combination with paclitaxel or vinorelbine were greater than the bliss index (Fig. 2B). In addition, caspase 3/7 activity in cells treated with a combination of YM155 with paclitaxel or vinorelbine was greater than the sum of that in cells treated with each single agent alone (Fig. 2C). In contrast, YM155 did not enhance the cell growth inhibition or caspase 3/7 activation induced by doxorubicin (Fig. 2).
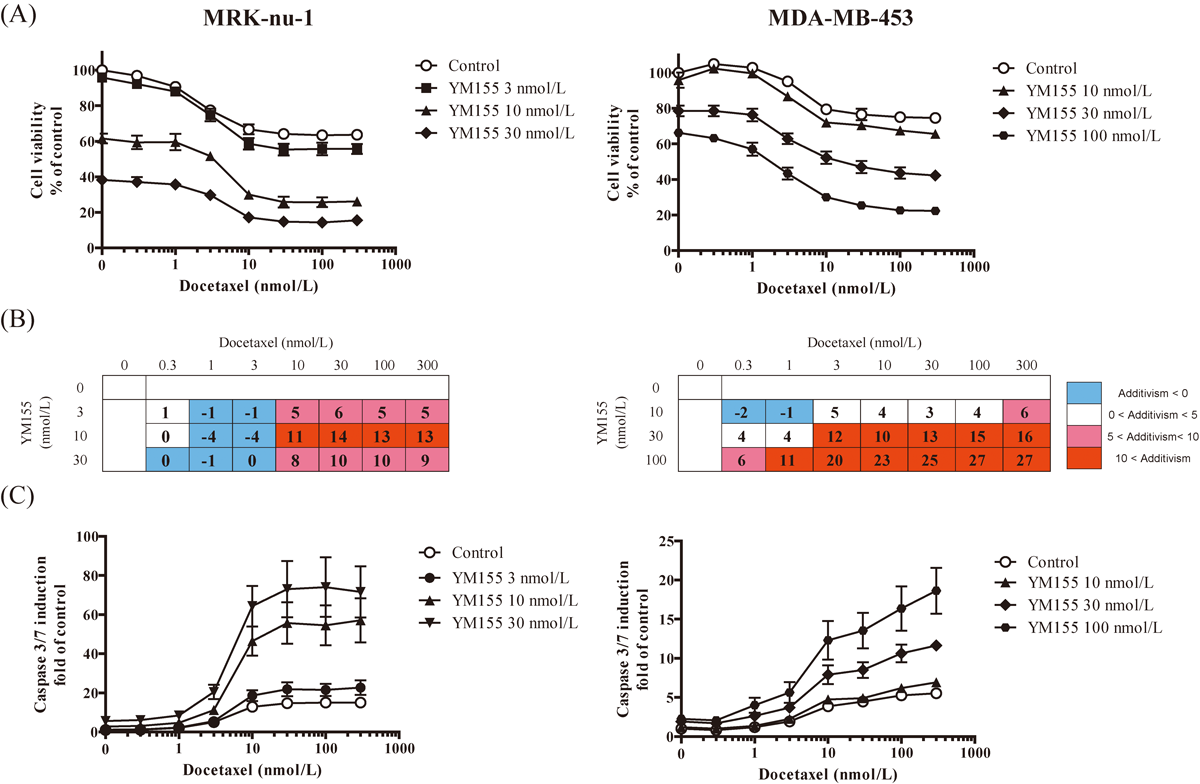
MRK-nu-1 cells and MDA-MB-453 cells were treated with docetaxel for 24 h and 48 h, respectively, followed by YM155 for 8 h. (A) Cell viability was determined using a CellTiter-Glo® Luminescent Cell Viability Assay. The luminescent value of treated cells was normalized to that of DMSO control and is shown as % inhibition of DMSO control. Each point represents the mean±S.E. from three independent assays. (B) Average observed excess inhibition (%) relative to that predicted by the Bliss additivism model from three independent assays. (C) Caspase 3/7 activity was determined using a Caspase-Glo3/7® assay. The luminescent value was normalized to that of cell viability and is shown as fold of the DMSO control. Each point represents the mean±S.E. from three independent assays.

MRK-nu-1 cells were treated with paclitaxel, vinorelbine or doxorubicin at the indicated concentrations for 24 h, then with YM155 (10 nmol/L) for 8 h. (A) Cell viability was determined using a CellTiter-Glo® Luminescent Cell Viability Assay. The luminescent value of treated cells was normalized to that of DMSO control and is shown as % inhibition of DMSO control. Each point represents the mean±S.D. of a representative assay. (B) The values are the observed excess inhibition (%) relative to that predicted by the Bliss additivism model from a representative assay. (C) Caspase 3/7 activity was determined using a Caspase-Glo3/7® assay. The luminescent value was normalized to that of cell viability and is shown as fold of the DMSO control. Bars represent means±S.D. of a representative assay.
Next, we assessed whether docetaxel affects the uptake of YM155 into MRK-nu-1 cells. No statistically significant difference was observed between the concentration of YM155 in cells with and without pretreatment with docetaxel. The mean concentrations of YM155 in MRK-nu-1 cells with or without pretreatment with docetaxel were 30 and 35 nmol/g protein, respectively (Table 1).
| Treatment | Docetaxel (−) | Docetaxel (+) | p-Value |
|---|---|---|---|
| YM155 concentration (nmol/g protein) | 30.1±1.15 | 35.3±1.94 | 0.0856 |
MRK-nu-1 cells were treated in triplicate wells with (+) or without (−) docetaxel at 100 nmol/L for 3 h followed by YM155 at 30 nmol/L for 4 h. Cells were harvested, and YM155 drug concentration was measured. Values were expressed as means±S.E. (n=3). The p-value was calculated by Student’s t-test.
To investigate the mechanisms responsible for the observed interaction between YM155 and microtubule-targeting agents, we examined changes in the expression of survivin and phosphorylated histone H3, an M-phase marker, as well as changes in cell cycle status in MRK-nu-1 cells. Treatment with docetaxel, paclitaxel, and vinorelbine for 24 h induced G2/M arrest and resulted in an accumulation of survivin and phosphorylated histone H3. In contrast, doxorubicin did not induce G2/M arrest and accumulation of survivin (Figs. 3A, B). On combination treatment, YM155 inhibited docetaxel-, paclitaxel-, and vinorelbine-induced accumulation of survivin and phosphorylated histone H3, as well as G2/M arrest, which resulted in an increased amount of cleaved PARP and an increased sub-G1 population than with either single agent alone (Figs. 3C, D).

MRK-nu-1 cells were treated with docetaxel (10 nmol/L), paclitaxel (10 nmol/L), vinorelbine (100 nmol/L), and doxorubicin (1 µmol/L) for 24 h. (A) Whole cell lysates were subjected to Western blotting using anti-survivin and anti-β-actin antibodies. (B) MRK-nu-1 cells were harvested and cell cycle distribution was determined by flow cytometry. MRK-nu-1 cells were treated with docetaxel (10 nmol/L), paclitaxel (10 nmol/L), and vinorelbine (100 nmol/L) for 24 h and then with or without YM155 (10 nmol/L) for 8 h. (C) Whole cell lysates were subjected to Western blotting using anti-survivin, anti-phospho histone H3 (ser 10), anti-cleaved PARP, and anti-β-actin antibodies. (D) Cells were harvested and cell cycle distribution was determined by flow cytometry.
We finally investigated whether combined treatment with YM155 and docetaxel might also exert a synergistic effect on TNBC tumor growth in vivo. YM155 and docetaxel alone induced tumor regression in a MRK-nu-1 xenograft model of up to 9% and 26%, respectively. Combined treatment with YM155 and docetaxel induced complete regression in 5 of 5 mice bearing MRK-nu-1 tumors (Table 2, Fig. 4A). Further, no significant enhancement of docetaxel-induced body weight loss was observed during 22 d (Fig. 4B).
| Treatment group | Antitumor activity (% reg) | Number of CR (CR/total mice) |
|---|---|---|
| Vehicle control | — | 0/5 |
| YM155 | 9% | 0/5 |
| Docetaxel | 26% | 0/5 |
| YM155+Docetaxel | 100% | 5/5 |
Percent tumor regression (% reg) was calculated using the following formula: 100×[1−(mean tumor volume of each group on day 22)/(mean tumor volume of each group on day 0)]. The number of complete regressions (CRs) in all groups was checked during the experimental period. CRs were defined as instances in which the tumor regressed to a size below the limit of palpation.

Values are expressed as means±S.E. (n=5). Mice received a continuous s.c. infusion of YM155 at 2 mg/kg/d for 7 d starting on day 0. Docetaxel was administered via an i.v. bolus injection at 20 mg/kg on day 0. Tumor volume and body weight on day 22 were compared between each single compound group and the combined group using Student’s t-test. *** p<0.001 versus YM155 group, ### p<0.001 versus the docetaxel group, N.S.: not significantly different from the YM155 or docetaxel group by Student’s t-test.
In this study, we investigated the combined effect of YM155 and docetaxel in TNBC models. In TNBC cells, the combination of YM155 with docetaxel decreased cell viability and increased caspase 3/7 activity to a greater extent than either single agent alone (Figs. 1A, C). Bliss additivism analysis revealed that the combined effect was synergistic (Fig. 1B). Similar results were observed when YM155 was combined with paclitaxel or vinorelbine (Fig. 2). These results suggest that YM155 synergistically potentiates the efficacy of microtubule-targeting agents in TNBC cells in vitro.
YM155 might potentiate the effect of microtubule-targeting agents by two mechanisms. The first one is drug–drug interaction. Pharmacokinetic analyses indicated that YM155 is highly distributed to tumors and reaches concentrations approximately 20-fold higher than those in plasma.25) Transportation of YM155 across the cell membrane likely involves the organic cation transporters OCT1 and OCT2 and multidrug resistance protein 1 (MDR1).26–28) MDR1 also effluxes taxanes and is related with drug resistance to taxanes.29) We therefore hypothesized that efflux of YM155 from cancer cells is affected by taxanes, resulting in increased concentrations of YM155 in cancer cells. The concentration of YM155 in MRK-nu-1 cells was not affected by docetaxel (Table 1). A similar result was obtained in MDR1-overexpressing MCF-7 cells (data not shown). These results suggest that the combined effect of YM155 and docetaxel does not arise from drug-drug interaction.
The second mechanism is survivin-mediated cytoprotection during G2/M arrest. We showed that all tested microtubule-targeting agents induced G2/M arrest and accumulation of survivin in MRK-nu-1 cells. In contrast, doxorubicin did not induce G2/M arrest and accumulation of survivin (Figs. 3A, B), and YM155 did not enhance the efficacy of doxorubicin (Fig. 2). These findings led to us to hypothesize that the elevated survivin expression in G2/M arrest caused by microtubule-targeting agents engenders apoptosis resistance. Survivin has been implicated in both cell survival and regulation of mitosis,9) and its expression is regulated in a cell cycle-dependent manner and peaks at mitosis.30) Survivin protects transformed cells from apoptosis. Further, interference with survivin causes spontaneous cell death and enhances cell death stimuli.31–33) Survivin is a component of the chromosomal passenger complex that is essential for proper chromosome segregation and cytokinesis. In addition, survivin is directly associated with polymerized tubulin and stabilizes microtubules.34) Survivin gene knockdown causes apoptosis in neuroblastoma via mitotic catastrophe.35) Here, we showed that YM155 inhibited microtubule-targeting agents-induced accumulation of survivin; decreased G2/M populations; and increased caspase-3/7 activity, PARP cleavage, and sub-G1 populations (Figs. 1C, 3C, D). These results suggest that survivin suppression by YM155 increases susceptibility to apoptosis and enhances the disruption of mitosis, resulting in an enhanced response to microtubule-targeting agents.
In addition, the combination of YM155 with docetaxel resulted in strong antitumor activity, achieving CR for all mice without exacerbation of body weight loss in a MRK-nu-1 TNBC xenograft model (Table 2, Fig. 4). In previous studies, we demonstrated that tumor regression induced by YM155 in combination with docetaxel was accompanied by a decrease in intratumoral survivin and an increase in apoptosis rate compared with either single agent alone in a human Calu-6 NSCLC xenograft model.21) We also demonstrated that YM155 reduced spontaneous metastases and significantly prolonged the survival of animals bearing human TNBC tumors.20) The dose of YM155 used in these studies were equivalent with the clinical dosing used and steady-state serum concentration observed in cancer patients. These results suggest that the therapeutic potential of YM155 is enhanced when administered in combination with microtubule-targeting agents in TNBC. In summary, we demonstrated the combination effect of YM155 with microtubule-targeting agents against TNBC models. These results support the conclusion that survivin inhibition might be an effective way to enhance the efficacies of microtubule-targeting agents against TNBC.