2013 Volume 36 Issue 12 Pages 1969-1974
2013 Volume 36 Issue 12 Pages 1969-1974
To clarify the relationship between selenium supplementation and type I allergic reaction, we investigated the effect of seleno-L-methionine (SeMet) supplementation on the active cutaneous anaphylaxis (ACA) reaction and cytokine production in splenocytes. Female BALB/c mice were sensitized by intraperitoneal injection of ovalbumin (OVA), and SeMet was administered orally for 2 weeks followed by a challenge with OVA to induce an ACA reaction. SeMet supplementation suppressed the ACA reaction in a dose-dependent manner. Plasma OVA-specific immunoglobulin E (IgE) level was strongly inhibited in SeMet-supplemented mice compared with control mice. The mRNA expression levels of the T helper 2 (Th2) cytokines interleukin (IL)-4 and IL-13 in the spleen of SeMet-supplemented mice were lower than those in control mice. The mRNA expression level of a Th1 cytokine, interferon (IFN)-γ, in the spleen of SeMet-supplemented mice was higher than that in control mice. Splenocytes restimulated with OVA in vitro from SeMet-supplemented mice produced lower amounts of IL-4 and IL-13 than those of control mice and higher amounts of IFN-γ than those from the control mice. These results suggest that oral SeMet supplementation suppresses OVA-induced ACA reaction by lowered Th2 cytokine production and augmenting Th1 cytokine production.
Allergic diseases have become a significant health problem, affecting more than 15% of children and adults worldwide, and their incidence is rising.1,2) Type I allergic diseases, such as atopic dermatitis, asthma and allergic rhinitis, are characterized by an elevated production of the specific immunoglobulin E (IgE) for each antigen.3) Naïve CD4+ T cells can differentiate into at least two different types of T helper (Th) cells, Th1 and Th2.4,5) Th2 cells are characterized by the production of interleukin (IL)-4 and IL-13, which promote the production of IgE. In contrast, Th1 cells are characterized by the production of interferon (IFN)-γ, which inhibits IgE production and the proliferation of Th2 cells.6–9) It is generally accepted that enhancement of Th2-mediated immunity causes IgE-dependent allergic diseases. Therefore, in the prevention and treatment of type I allergic diseases, it is considered important that the Th2-polarized immune response shifts toward a Th1 phenotype.
Selenium is an essential trace element for mammals and many other forms of life.10) Selenium has been shown to regulate many intracellular functions by being a chemical component of selenoproteins. Well-known selenoproteins include enzymes such as glutathione peroxidase (GPx) and thioredoxin reductase (TR), which have a selenocysteine residue in their catalytic centers and function as critical enzymes in response to oxidative stress.11) The primary dietary selenium sources are wheat12) and yeast,13) which contain seleno-L-methionine (SeMet) and/or selenomethylselenocystine. Many nutritional studies on selenium have been conducted with inorganic selenium compounds, such as sodium selenite and sodium selenate. However, organic selenium compounds, SeMet and selenocystine, have excellent bioavailability and are more appropriate for nutritional supplementation compared with inorganic selenium compounds.14–17) Moreover, SeMet has lower toxicity compared with various selenium compounds.18–21)
Some studies have suggested that patients with type I allergic diseases, such as atopic dermatitis and asthma, had significantly lower selenium concentrations in hair or blood, and significantly lower GPx activity in blood.22–25) This has led to speculation that supplementation of selenium compounds might be effective for prevention of type I allergic diseases. Although it has been reported that intraperitoneal (i.p.) injection of sodium selenite in an experimental mouse model of asthma suppresses the activity of nuclear factor (NF)-κB and inhibits infiltration of eosinophils into lung airway,26) it remains unclear whether oral supplementation of SeMet would be beneficial for preventing type I allergic reactions.
In this study, to clarify the relationship between selenium supplementation and type I allergic reactions, we administered SeMet to ovalbumin (OVA)-sensitized mice and investigated the active cutaneous anaphylaxis (ACA) reaction, which is an experimental mouse model of type I allergy. We revealed that SeMet supplementation suppressed the ACA reaction and OVA-specific IgE elevation elicited by repeated OVA injection of mice. We also examined whether SeMet affects the mRNA expression levels of Th2 cytokine IL-4 and IL-13 and the Th1 cytokine IFN-γ in the spleen, and cytokine production of IL-4, IL-13 and IFN-γ in splenocytes from OVA-sensitized mice restimulated with OVA in vitro.
Female five-week-old BALB/c mice were purchased from Japan SLC Co. (Shizuoka, Japan) and rested for 1 week after arrival. The animals were treated and kept in a specific pathogen-free room maintained at 23±1°C and 47–67% humidity, under a 12 h light–dark cycle. Mice were given a normal diet (Type CRF-1, Oriental Yeast Co., Tokyo, Japan) throughout the experiment. The experimental protocol satisfied the Animal Experimental Guidelines of Setsunan University, which were modified from the guidelines of the Japanese Society for Pharmacology. This experiment was approved by the Committee for the Ethical Use of Experimental Animals of Setsunan University.
ReagentsSeMet was purchased from Acros Organics (Geel, Belgium). OVA (five times crystalized) was purchased from Seikagaku Co. (Tokyo, Japan). Aluminum hydroxide hydrate gel suspension (alum) was purchased from Cosmo Bio, LSL (Tokyo, Japan). Evan’s blue was purchased from Tocris Bioscience (MO, U.S.A.).
OVA-Sensitization and Challenge, and SeMet SupplementationOn day 0 and day 7, mice were sensitized with i.p. injection of 1 µg of OVA and 1 mg of alum dissolved in 0.1 mL of saline. SeMet (5 or 10 µmol/kg/d) or saline was administered orally once daily on days 0–14. On day 14, ACA was performed as described by Inagaki et al.,27) with a slight modification. In brief, the mice were challenged with 1 µg OVA in saline by subcutaneous (s.c.) injection into the ear followed by intravenous (i.v.) injection of 0.25 mL of 0.5% Evan’s blue solution. Thirty minutes after the challenge, the mice were sacrificed and their ears, plasma and spleen were collected. The ears were used to measure the extravasated dye. Extraction and quantification of the extravasated dye was performed as described by Inagaki et al.28) The amount of dye was measured colorimetrically at 620 nm.
Measurement of Selenium ConcentrationThe plasma (0.1 mL), liver and spleen tissues (0.05g) were digested at 80°C for 1 h, then 140°C for 2 h and then 170°C for 30 min in 1 mL of a 1 : 2 mixture of nitric acid and perchloric acid. The selenium concentration was measured by the fluorometric method using 2,3-diaminonaphthalene.29)
Measurement of OVA-Specific IgE in PlasmaOVA-specific IgE in plasma was measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available mouse OVA-specific IgE ELISA Kit (MD Bioproducts, Zürich, Switzerland). The absorbance was measured at 450 nm using a microplate reader.
Cytokine mRNA Analysis of SpleenTotal RNA was extracted from an aliquot of spleen with Sepasol reagent (Nacalai Tesque, Kyoto, Japan) in accordance with the manufacturer’s instructions. The concentration and purity of extracted RNA were determined using A260/A280 measured on NanoDrop 1000 spectrometer (Thermo Fisher Scientific, MA, U.S.A.). Synthesis of cDNA was conducted using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, U.S.A.). The LightCycler 480 System II (Roche Diagnostics GmbH, Mannheim, Germany) was used for real-time quantitative polymerase chain reaction (PCR) analysis. The oligonucleotides used for quantitative PCR included IL-4 (forward: 5′-TCT CGA ATG TAC CAG GAG CCA TAT C-3′, reverse: 5′-AGC ACC TTG GAA GCC CTA CAG A-3′), IL-13 (forward: 5′-CCT CTG ACC CTT AAG GAG CTT AT-3′, reverse: 5′-CGT TGC ACA GGG GAG TCT-3′), IFN-γ (forward: 5′-CGG CAC AGT CAT TGA AAG CCT A-3,′ reverse: 5′-GTT GCT GAT GGC CTG ATT GTC-3′), Rps18 (forward: 5′-TTC TGG CCA ACG GTC TAG ACA AC-3′, reverse: 5′-CCA GTG GTC TTG GTG TGC TGA-3′). Cycling conditions were used as suggested in the SYBR Green Kit instructions, and results were analyzed using Relative Quantification Software (Roche Diagnostics GmbH). The results were expressed as mean±S.D. for the relative expression levels compared with Rps18.
Splenocyte Culture and Cytokine ELISASplenocytes were prepared by the following method. An aliquot of spleen was gently homogenized and through mesh. After lysis of red blood cells using ammonium chloride hematopoietic buffer, splenocytes were suspended in RPMI medium supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. The cells were cultured for 72 h at 37°C, 5% CO2 in the presence of 100 µg/mL OVA. The cell culture supernatants were stored at −80°C until analysis by ELISA. The amounts of IL-4, IL-13 and IFN-γ in the cell culture supernatants were measured by ELISA kits (IL-4 and IFN-γ: BioLegend, CA, U.S.A.; IL-13: eBioscience, CA, U.S.A.), which was performed in accordance with the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader.
Statistical AnalysisStatistical significance between two groups was examined using unpaired Student’s t-test. The symbols * and ** indicate statistical significance at p values of 0.05 and 0.01 in Table and Figs. 1–4, respectively.
In order to clarify the relationship between selenium supplementation and ACA reaction, SeMet or saline (vehicle control) was administered orally to BALB/c mice with ACA for 2 weeks. Body weight, coat appearance and general grooming were not significantly different between the SeMet-supplemented and control groups (data not shown). As shown in Table 1, the selenium concentration in plasma, liver and spleen was increased by SeMet supplementation in a dose-dependent manner. The effect of SeMet supplementation on type I allergy was measured using the ACA reaction. Assessment of the ACA reaction was done using the amount of extravasated dye, which indicates the permeability of blood vessels at a site of inflammation. As shown in Fig. 1, SeMet supplementation suppressed the amount of extravasated dye in a dose-dependent manner. The amount of extravasated dye in mice administered 5 µmol/kg/d of SeMet decreased to 67.2% of that of the control group. In mice administered 10 µmol/kg/d of SeMet, the amount was suppressed significantly to 38.7% of that in the control group. Because the ACA reaction was suppressed significantly at a dose of 10 µmol/kg/d, we focused on this for subsequent experiments.
The production of antigen-specific IgE is a crucial component of type I allergy. Plasma was collected at 30 min after the challenge and measured by ELISA. As shown in Fig. 2, the OVA-specific IgE level in plasma was strongly inhibited to 38.6% in SeMet-supplemented mice, compared with the control group.
| SeMet (µmol/kg/d) | Plasma (ng/mL) | Liver (ng/g) | Spleen (ng/g) |
|---|---|---|---|
| 0 | 339.06±15.76 | 1622.55±349.58 | 485.84±54.02 |
| 5 | 457.86±83.77* | 3376.98±269.84** | 955.69±69.49** |
| 10 | 528.16±104.69** | 4937.93±1291.45** | 1456.15±276.78** |
The results are presented as mean±S.D. (n=10). ** p<0.01, * p<0.05 compared with control.
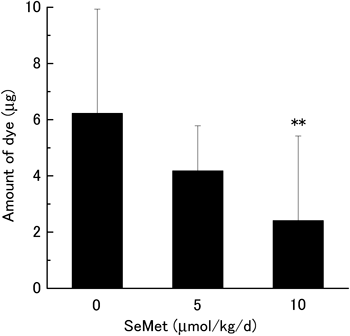
Mice were supplemented with SeMet for 2 weeks and sensitized with OVA/alum by i.p. injection twice. On the final day, the mice were challenged with OVA by s.c. injection into the ear followed by i.v. injection of Evan’s blue solution. The amount of extravasated dye was measured colorimetrically at 620 nm. The results are presented as mean±S.D. (n=15). ** p<0.01 compared with control.

Mouse plasma was obtained from each mouse and the OVA-specific IgE level was determined by ELISA. The results are presented as mean±S.D. (n=10). * p<0.05 compared with control.
In type I allergy, the immune response is polarized to a Th2 phenotype. To examine the effect of SeMet supplementation on CD4+ T cell differentiation, spleen was collected at 30 min after the challenge and total RNA was extracted. The mRNA expression levels of IL-4 and IL-13 (Th2 cytokines) and IFN-γ (Th1 cytokine) were measured using real-time quantitative PCR. As shown in Figs. 3A and B, the mRNA expression levels of IL-4 and IL-13 were significantly lower in SeMet-supplemented mice. Conversely, the mRNA expression level of IFN-γ was significantly higher in SeMet-supplemented mice (Fig. 3C). The mRNA expression levels of IL-4 and IL-13 in unsensitized mice were significantly low compared with the OVA-sensitized control mice (Figs. 3A, B). The mRNA expression level of IFN-γ in unsensitized mice was the same level of the OVA-sensitized control mice (Fig. 3C).

Total RNA was extracted from the spleen, and IL-4 (A), IL-13 (B) and IFN-γ (C) mRNA expression levels were determined by real-time quantitative PCR. The results are presented as mean±S.D. (n=10) for the relative expression levels compared with Rps18. ** p<0.01, * p<0.05 compared with control.
To examine the cytokine production ability of splenocytes from SeMet-supplemented mice, splenocytes was collected at 30 min after the challenge and cultured for 72 h with OVA in vitro. As shown in Fig. 4A, the splenocytes from OVA-sensitized, SeMet-supplemented mice restimulated with OVA released significantly lower levels of IL-4 in mice compared those from control mice. IL-13 produced by the splenocytes of the SeMet-supplemented mice was likely to be lower than that in the control (Fig. 4B). However, the level of IFN-γ produced by the splenocytes was significantly higher in SeMet-supplemented mice compared with the control (Fig. 4C).

Splenocytes were cultured with OVA for 72 h, the supernatants were collected and production levels of IL-4 (A), IL-13 (B) and IFN-γ (C) were determined by ELISA. The results are presented as mean±S.D. (n=10). ** p<0.01, * p<0.05 compared with control.
Patients with type I allergic diseases, such as atopic dermatitis and asthma, show significantly lower selenium concentrations in hair or blood.22–25) This had led to speculation that supplementation of selenium compounds might be effective to prevent type I allergic diseases. It has been reported that i.p. injection of sodium selenite in an experimental mouse model of asthma suppresses the activity of NF-κB and inhibits infiltration of eosinophils into lung airway.26) However, it remains unclear whether oral supplementation of SeMet would be beneficial for preventing type I allergic reactions. Furthermore, the effect of SeMet supplementation on the production of Th1 and Th2 cytokines in splenocytes is not known. In this study, we showed that oral SeMet supplementation suppressed the OVA-induced ACA reaction by lowering Th2 cytokine production and augmenting Th1 cytokine production.
In the present study, 5 µmol/kg/d of SeMet administered to mice tended to decrease the ACA reaction compared with the control group. In mice receiving 10 µmol/kg/d of SeMet exhibited a significantly suppressed ACA reaction. The suppression level of the ACA reaction by SeMet supplementation was dose-dependent.
Type I allergic diseases, such as atopic dermatitis and asthma, are characterized by the elevated production of an antigen-specific IgE.3) In this study, OVA-specific IgE level in the plasma of SeMet-supplementated mice was significantly lower than in mice in the control group. These results suggest that SeMet supplementation affects the production of antigen-specific IgE. Secretion of IgE is induced by the activation of Th2 cells, and the activation of Th2 cells is regulated by Th1 cells.6–9) Sensitization by intraperitoneal injection of OVA and alum shifts the Th1/Th2 response toward Th2. Therefore, attempts to exaggerate the Th1 response are important for such Th2-biased immune reactions to restore Th1/Th2 balance. To assess the effect of SeMet on CD4+ T cell differentiation, total RNA was extracted from the spleen, and the mRNA expression levels of Th2 cytokines (IL-4, IL-13) and a Th1 cytokine (IFN-γ) were measured. The IL-4 and IL-13 mRNA expression levels were significantly lower in mice receiving SeMet supplementation than in control mice. Conversely, the mRNA expression of IFN-γ was significantly higher in mice receiving SeMet supplementation than in control mice.
It has been reported that there is little production of IL-4, IL-13 and IFN-γ by splenocytes from OVA-sensitized mice cultured for 72 h without OVA.30,31) Therefore, we investigated the effect of SeMet on the production of IL-4, IL-13 and IFN-γ by splenocytes from OVA-sensitized mice cultured for 72 h with OVA in vitro. Splenocytes from the SeMet-supplemented group exhibited a significantly lower production of IL-4 than those from the control group. Similarly, IL-13 production showed a tendency to decrease in splenocytes from the SeMet-supplemented group compared with those from the control group. IFN-γ production in splenocytes from the SeMet-supplemented group increased significantly compared with those from the control group. Cytokine production in splenocytes restimulated with OVA in vitro correlated with the cytokine mRNA expression levels in the spleen. Elser et al. reported that IFN-γ suppresses IL-4 expression levels at the transcriptional level via the repression of IL-4 promoter activity by IFN-γ-inducible factors IRF-1 and IRF-2.32) These findings suggest that SeMet supplementation might regulate the expression of IL-4 and IFN-γ at the transcriptional level.
It has been reported that plasma selenium concentration is useful in monitoring compliance of selenium supplementation with SeMet.15) We measured plasma selenium concentrations of SeMet-supplemented and control mice. The selenium level in the plasma of mice supplemented with 5 or 10 µmol/kg/d SeMet increased 1.3-fold and 1.6-fold compared with control mice, respectively. The selenium content in the liver and spleen was also increased by SeMet supplementation in a dose-dependent manner. Absorbed selenium is used for the synthesis of selenoproteins such as GPx, TR and selenoprotein S (SelS). GPx and TR are anti-oxidant enzymes and their activities are significantly increased in mice or rats fed high supplemental level of selenium containing diet compared with mice or rats fed normal level of selenium containing diet.33–35) Recently, it was reported that increasing GPx and TR activities alter the immune response toward a Th1 phenotype.36) SelS is also considered to regulate the immune response and redox balance.37,38) These findings suggest that SeMet supplementation might optimize Th1 and Th2 cytokine expression by regulation of redox balance through increased production of selenoproteins.
In the present study, SeMet or saline was administered orally to BALB/c mice for 2 weeks. There were no significant differences in food consumption between control group and SeMet-supplemented group (data not shown). According to the manufacturer’s data, selenium concentration in the diet was 0.42 ppm. Therefore, in this study, each mouse received an average of 1.0 µg/d of selenium from normal diet. The mean values of selenium intake by oral administration of SeMet were 7.4 µg selenium/mouse/d for 5 µmol/kg/d of SeMet-supplemented group, and 14.9 µg selenium/mouse/d for 10 µmol/kg/d of SeMet-supplemented group. It has been reported that oral administration of SeMet at 0.2 mg/mouse/d (80.5 µg selenium/mouse/d) for 28 d is safe and nontoxic for mice.39) Therefore, the administration dose in this study is considered to be safe and nontoxic for mice. The U.S. Food and Nutrition Board at the Institute of Medicine set the recommended daily allowance (RDA) of selenium at 55 µg/d for adult human.40) The dietary requirement of selenium for mice is 0.4 µg/d, according to the National Research Council.41) The daily requirement of selenium for human is much lower than mice, further studies are required for clinical application.
In conclusion, we have shown that oral SeMet supplementation suppressed OVA-induced ACA reaction in mice, which is mediated by inhibition of OVA-specific IgE elevation elicited by repeated OVA injection, a decrease in Th2 cytokines (IL-4, IL-13) production, and the augmentation of Th1 cytokine (IFN-γ) production.
The authors thank Ms. Aoi Fujita, Mr. Yuki Hirota, Ms. Yurie Yoshii, Ms. Shizuka Nakayama, Ms. Airi Minamide and Ms. Mariko Tatsu for excellent technical assistance.