2015 Volume 38 Issue 1 Pages 109-115
2015 Volume 38 Issue 1 Pages 109-115
Cinnamomum cassia is widely employed for gastrointestinal complaints such as dyspepsia, flatulence, diarrhea, and vomiting. Studies report cinnamaldehyde (CM) as a major active constituent of cinnamon. The aim of this study was to evaluate the anti-inflammatory mechanism of CM on Helicobacter (H.) pylori-infected gastric epithelial cells in order to validate cinnamon traditional use in gastrointestinal (GI)-related disorders. AGS/MKN-45 cells and H. pylori (193C) were employed for co-culture experiments. Anti-H. pylori cytotoxic and anti-adhesion activity of CM were determined. Enzyme linked immunosorbent assay, real time polymerase chain reaction analysis and immunoblotting were used to measure the effect on interleukin-8 (IL-8) secretion/expression. The effect on activation of nuclear factor kappa B (NF-κB) was determined by immunoblot analysis. The non-cytotoxic CM (≤125 µM) was also non-bactericidal at the given time, suggesting the effect in H. pylori/cell co-culture system was not due to alteration in H. pylori viability or the toxicity to the cells. Also, CM did not show any anti-adhesion effect against H. pylori/cell co-culture. However, pre-incubation of the cells with CM significantly inhibited the IL-8 secretion/expression from H. pylori-infected cells (p<0.01). In addition, CM suppressed H. pylori-induced NF-κB activation and prevented degradation of inhibitor (I)-κB This study provides evidence that the anti-inflammatory effect of C. cassia on H. pylori-infected gastric cells is due to blockage of the NF-κB pathway by cinnamaldehyde. This agent can be considered as a potential candidate for in vivo and clinical studies against various H. pylori related gastric pathogenic processes.
Helicobacter pylori colonizes approximately 50% of the world population and is considered as the most common chronic bacterial infection in humans.1,2) H. pylori infection leads to several pathologic alterations in stomach, including atrophic gastritis, peptic ulcer, and gastric adenocarcinoma.3) A recent epidemiological study reveals high H. pylori prevalence and strong linkage to development of gastric cancer in developing Asian countries.4) In fact, the International Agency for Research on Cancer (IARC) declared H. pylori to be a group I human carcinogen for gastric adenocarcinoma.5)
The regimens recommended for the treatment of H. pylori are triple therapy and quadruple therapy.6) However, this initial attempt of eradicating H. pylori fails in approximately 20–30% of the patients.7) Emerging bacterial resistance to antibiotics limits their use in the treatment of infections.8,9) Therefore, search for non-antibiotic substances for the treatment of H. pylori associated gastric diseases is crucial.10,11)
Although H. pylori is a non-invasive organism, it stimulates a robust inflammatory and immune response. The net result is increased production of inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and most notably, interleukin-8 (IL-8). IL-8 is a potent chemotactic factor, activates neutrophils, and recruits acute inflammatory cells into the mucosa.12) H. pylori appear to activate nuclear factor kappa B (NF-κB), which in turn increases IL-8 production.13) Another hallmark of the infection with H. pylori is the formation of hummingbird phenotype in the cells which correlates with the altered motility, migration, and adhesion of the cells.14) Therefore, a newer agent that can modulate production of IL-8 in H. pylori infected cells might propose an effective strategy to prevent H. pylori-induced pathological disorders.
In traditional system of Unani medicine, Cinnamomum cassia is widely employed in gastrointestinal and digestive complaints such as dyspepsia, gastritis, flatulence, diarrhea and vomiting.15–18) Although various biological activities including anti-inflammatory activites of C. cassia have been reported, to the best of our knowledge there is no report on its molecular mechanism against H. pylori-induced inflammation. Also previous studies report cinnamaldehyde (CM) as an active constituent of C. cassia.19) Therefore, we hypothesize that anti-inflammatory effect of C. cassia is due to CM.
We have previously reported IL-8 inhibitory activity of C. cassia in H. pylori-infected cells.20) In this present study, we examined the anti-inflammatory effect and elaborated molecular mechanism of CM by evaluating its effect on IL-8 production and NF-κB activation on H. pylori-infected gastric epithelial cells.
H. pylori clinical isolate 193C was cultured in Brucella broth (BBL™, BD, Franklin Lakes, U.S.A.) medium supplemented with 10% fetal bovine serum (FBS).21) The bacteria were sub-cultured, before co-culture experiments, for 24–48 h under microaerophilic conditions (5% O2, 10% CO2, and 85% N2 at 37°C; Sanyo-Multigas Incubator, SANYO Electric Co., Ltd., Tokyo, Japan) on a gyratory shaker at 160 rpm with 100% humidity. The bacterial concentration was estimated by using the formula as an absorbance of 0.1=108 bacteria/mL.22)
Determination of Non-bactericidal ConcentrationAnti-H. pylori effects of C. cassia has been evaluated previously.20) In this study non-bactericidal concentration of CM (Sigma-Aldrich, Japan) was evaluated by the method described with minor modifications.23) Briefly, H. pylori were either left untreated or treated with CM diluted in dimethyl sulfoxide (DMSO) at various concentration range for 1 h at 37°C. Bacteria were then serially diluted and inoculated onto commercial selective Pylori agar plates (Kyokuto; Tokyo, Japan) under microaerophilic conditions as presented above. After the incubation of 2–3 d, the bacterial colonies were counted and the colony forming units (CFUs) were calculated. Data are expressed as percent of survival. The results are representative of three independent experiments.
Cell Survival Study by DNA Fragmentation AssayQuantitative DNA fragmentation assay was carried out as given previously.24) Briefly, cells pretreated with or without CM were lysed in a lysis buffer (10 mmol⁄ L Tris, 1 mmol⁄L ethylenediaminetetraacetic acid (EDTA), 0.2% Triton X-100, and pH 7.5) and centrifuged at 13000×g for 10 min. Then, each DNA sample in the supernatant and the resulting pellet were precipitated in 12.5% trichloroacetic acid (TCA) at 4°C, and quantified using a diphenylamine reagent after hydrolysis in 5% TCA at 90°C for 20 min. The percentage of fragmented DNA for each sample was calculated as the amount of DNA in the supernatant divided by the total DNA for that sample (supernatant plus pellet).
Experimental Design for Co-culture ExperimentsHuman gastric epithelial cell lines (AGS and MKN-45) (gastric adenocarcinoma, ATC C CRL 1739) were purchased from the American Type Culture Collection (Rockville, MD, U.S.A.). The cells were grown in RPMI 1640 (Wako Chemical Industries, Ltd., Osaka, Japan) containing 2 mmol/L M-glutamine supplemented with antibiotics and 10% FBS at 37°C in 5% CO2. Cells were routinely passaged every 3 d. Cells were seeded into 6 cm culture dish and grown for overnight followed by washing with phosphate-buffered saline (PBS) three times. The medium RPMI 1640, without antibiotics and FBS, was added. AGS cells, treated with or without CM (62.5 and 125 µM) for 30 min, then incubated in the presence of H. pylori at a bacterium/cell ratio of 50 : 1 for 4 h for mRNA expression, 6 h IL-8 secretion assay and Western blotting.
Anti-adhesion Activity AssayMKN-45 cells, derived from human gastric carcinomas, was used for the analysis of H. pylori adhesion by the method described previously.25,26) Briefly, cells were preincubated with or without CM (31.2–125 µM) in 96-well plates at 37°C under 5% CO2 for 60 min and H. pylori was added in the treated and untreated wells. The cells were then fixed at 4°C for 60 min and after washing, 100 µL of rabbit anti-H. pylori polyclonal antibody (Dako Cytomation, Glostrup, Denmark) was added to each well, and the plates were incubated for 2 h at 37°C. After washing, 100 µL of peroxidase-conjugated goat anti-rabbit Igs (Wako Pure Chemical Industries, Ltd.) diluted 1 : 1000 in PBS was added to each well and incubated for 2 h at 37°C. After final washing, 100 µL of substrate reagent pack (DY999; R&D System Inc., Minneapolis, MN, U.S.A.) was added for 15 min followed by addition of 100 µL of 1 mol/L H2SO4 to terminate the reaction. The optical density (OD) of the reaction was measured at 490 nm with a microplate reader. The OD represents the amount of H. pylori adhering to target cells.
Enzyme Linked Immunosorbent Assay (ELISA) for Effect on IL-8 SecretionAGS cells were co-cultured with H. pylori at multiplicity of infection (MOI) of 50 : 1 for 6 h in the presence or absence of CM. IL-8 secretion in the supernatant from the treated cells was analyzed by using ELISA (R & D System). After 6 h of culture, the supernatant medium was collected and IL-8 contents were determined according to the manufacturer’s instructions. A standard curve of recombinant IL-8 (R & D) was employed to determine IL-8 concentrations which are expressed in pg/mL.
Quantitative Analysis of IL-8 Expression by Real Time Polymerase Chain Reaction (RT-PCR)For the evaluation of IL-8 gene expression, cells were co-cultured with H. pylori in the presence or absence of CM for 4 h, then washed thrice with PBS, scrapped by rubber policeman and the total RNA was extracted by RNeasy Plus Mini kit (Qiagen, Germany) according to the manufacturer’s instructions. Reverse transcription was performed using the Exscript RT reagent kit (TaKaRa Bio Inc., Shiga, Japan) and random primers, followed by RT-PCR. Amplification of IL-8 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using SYBR Premix Ex Taq (TaKaRa Bio Inc.). The following primer pairs were used: Forward 5′-ACA CTG CGC CAA CAC AGA AAT TA-3′ and Reverse 5′-TTT GCT TGA AGT TTC ACT GGC ATC-3′ for IL-8; and, Forward 5′-GCA CCG TCA AGG CTG AGA AC-3′ and Reverse 5′-TGG TGA AGA CGC CAG TGG A-3′ for GAPDH. Real-time (RT)-PCR was carried out using the Mx3000 QPCR system (Agilent Tech.). IL-8 mRNA was normalized to GAPDH mRNA as an internal control in each sample. Results were expressed as the ratio relative to the average of uninfected cells.
Immunoblot AnalysisAGS cells were pretreated with CM for 30 min later infected with H. pylori for 6 h to assess IL-8 and for 45 min to assess NF-κB activation. H. pylori co-cultured AGS cells both untreated and treated with various concentration of CM were homogenized in 100 µL of cell lysis buffer (25 mmol/L of N-(2-hydroxyethyl)perazine-N′-2-ethanesulfonic acid (HEPES) pH 7.7, 0.3 mol/L of NaCl, 1.5 mmol/L of MgCl2, 0.2 mmol/L of EDTA, 0.1% Triton X-100, 20 mmol/L of β-glycerophosphate, 0.1 mmol/L of sodium orthovanadate, 0.5 mmol/L of phenylmethylsulfonyl fluoride, 1 mmol/L of dithiothreitol, 10 µg/mL of aprotinin, and 10 µg/mL of leupeptin). Protein concentration was determined by Bio-Rad DC Protein Assay (Life Science Research, U.S.A.). Twenty micrograms of protein was loaded per lane, separated by SDS-polyacrylamide gel electrophoresis (PAGE) under reducing condition, and transferred onto Immobilon-P nylon membrane (Millipore, MA, U.S.A.). The membrane was treated with BlockAce (Dainippon Pharmaceutical Co., Ltd., Suita, Japan) overnight at 4°C and probed with antibodies against IL8, NF-κB p65 (C-20-G), IκBα (C-21) and actin (C-11) from Santa Cruz Biotechnology (CA, U.S.A.) and phospho-NF-κB p65 (Ser536;93H1) from Cell Signaling Technology (Boston, MA, U.S.A.). The primary antibodies were detected using horseradish peroxidase conjugated anti-rabbit, or anti-goat immunoglobulinG (IgG) (Dako) and visualized with the enhanced chemiluminescent system (Amersham Biosciences, Buckinghamshire, U.K.).
Statistical AnalysisThe results are expressed as the mean±S.D. Quantification of Western blotting data for NF-κB pp65 relative to actin was performed using ImageJ software. Later statistical significance (*p<.01, **p<.05) was evaluated for PCR and NF-κB pp65 immunoblot quantification data by one-way ANOVA followed by Bonferroni post-hoc test.
Minimum bactericidal concentration value for ethanol extract of C. cassia against clinical isolates of H. pylori was previously reported by our group (≥ 500 µg/mL).27) Later on, five times lower to this concentration (100 µg/mL) was also shown to have little effect on bacterial viability.15) To determine non-bactericidal concentration and the effect of CM in H. pylori-infected cells, we analyzed the viability of H. pylori in the presence of CM at different concentrations. The results showed significant effect of CM on H. pylori viability at the concentrations of 500 µM. However, no significant effect was seen at 250 µM (Fig. 1a).
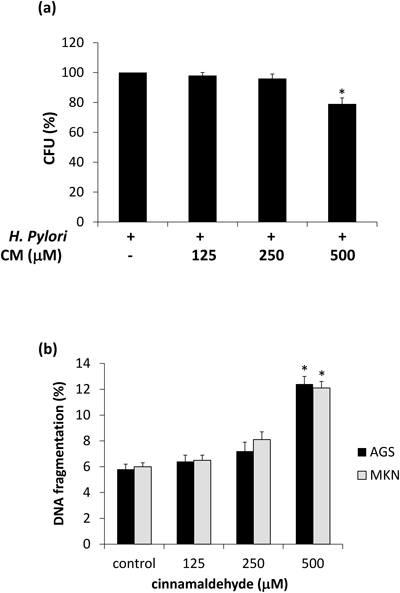
H. pylori viability is not significantly affected by (a) CM at concentration ≤250 µM, (b) CM at ≤250 µM had mild but non-significant cytotoxic effect on both AGS and MKN-45 cells. Higher drug concentration showed significant effect on bacterial viability and cytotoxic effect as compared to control. * p<.05 (compared to control) (n=3).
DNA fragmentation, a characteristic feature of apoptosis, was measured in a concentration dependent manner at 6 h of incubation with CM. The results revealed mild but non-significant induction of DNA fragmentation on concentrations of CM ≤250 µM (Fig. 1b) when compared with untreated cells. Hence, for later experiments a lower concentration of CM (≤ 125 μM) was used. These concentrations are selected to ensure that gastric cells and H. pylori are not directly affected by CM.
Anti-adhesion Activity against H. pylori Binding to Gastric Epithelial CellsColonization of gastric epithelial cells by H. pylori is the initial step leading to inflammatory changes. Later on, binding of H. pylori causes injection of various virulent proteins (e.g., CagA) directly into the gastric epithelial cells by type IV secretion system. Previously it has been demonstrated that an agent that could inhibit this binding of H. pylori to gastric epithelial cells might hinder H. pylori-associated gastric pathogenesis.26) To investigate whether the anti-inflammatory effect of CM is due to anti-adhesion activity we evaluate different non-bactericidal concentrations of CM (≤125 µM) by ELISA on gastric cancer cell line MKN-45. The results revealed that adhesion of H. pylori to gastric epithelial cells was not inhibited by CM pretreatment (Fig. 2).
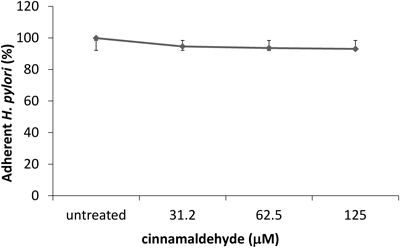
Cells were treated with various concentrations of CM (≤ 125 µM) for 60 min. The amount of adherent H. pylori is expressed as the percentage of the amount of H. pylori adhering to untreated cells. Each value represents the mean±S.D. (n=3).
Based on above these results we can speculate that any anti-inflammatory effect of CM (≤ 125 µM) and its extracts on gastric epithelial cells is neither a result of bacterial viability alteration nor by inhibition of bacterial adhesion.
Effect of Cinnamaldehyde on H. pylori-Induced IL-8 SecretionIL-8 is a crucial chemokine responsible in mediating H. pylori-induced inflammatory response in gastric mucosa.28) Previously we explored pharmacological basis of twenty four Pakistani medicinal plants by examining their effect on IL-8 secretion in H. pylori-infected gastric epithelial cells. At a concentration of 50 µg/mL, C. cassia showed strongest inhibitory activity against IL-8 secretion in H. pylori-infected gastric cells.20)
In this study we evaluated whether this anti-inflammatory effect of C. cassia is due to CM, its major constituent, or not. To determine anti-inflammatory activity of CM, low non-bactericidal concentrations (≤ 125 µM) of CM were used. As shown in Fig. 3, a strong increase in IL-8 content was observed in H. pylori-infected cells in the absence of any drug compared to the uninfected cells which was suppressed by CM in a concentration-dependent manner. At 125 µM of CM, the suppression of IL-8 secretion was maximum (p<.01) while mild suppression was seen at 62.5 µM concentration. CM alone has no effect on IL-8 production.
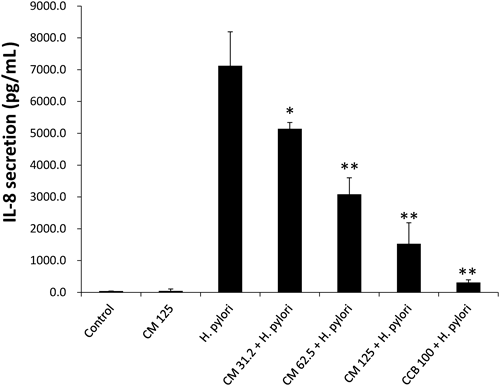
Cells were pretreated with concentrations of CM (31.2–125 µM) and Cinnamomum cassia (CCB: 100 µg/mL; positive control) and supernatants from H. pylori-co-cultured cells were analyzed for IL-8 content. Each value represents the mean±S.D. (n=3). ** p<.01, * p<.05 (compared to H. pylori-infected cells).
Expression of IL-8 is shown to be up-regulated via NF-κB activation in H. pylori-infected gastric epithelial cells.29) Hence, firstly we evaluated the effect of CM on IL-8 expression in H. pylori infected cells. In this study, overexpression of IL-8 was noted in H. pylori infected cells after 6 h of co-culture experiment. This up-regulation of IL-8 was significantly inhibited by increasing dose of CM (Fig. 4a). Measurement of relative mRNA expression shows up to ten times down regulation of IL-8 by 125 µM of CM (p<.01). Similar pattern of IL-8 down regulation is seen at protein level (Fig. 4b).
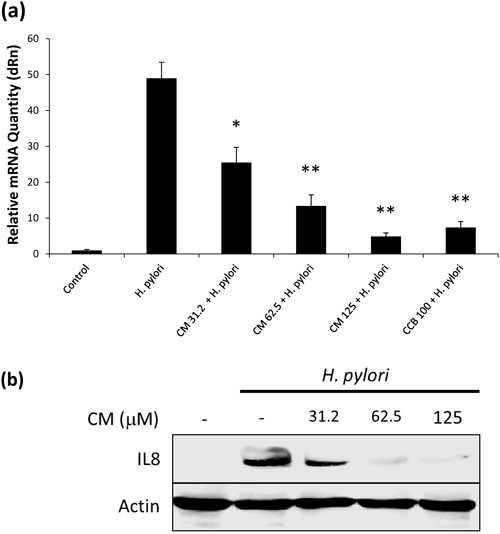
Cells incubated with or without CM (31.2–125 µM) for 60 min followed by the addition of H. pylori for 4 h. IL-8 mRNA expression and total cellular protein was measured. Results for (a) mRNA expression are expressed as the ratio relative to the uninfected cells and (b) Western blotting figure shows dose dependent effect of CM on IL-8 production after H. pylori infection for 6 h. Data represents the mean values of three independent experiments; ** p<.01 and * p<.05 (compared with untreated H. pylori-infected cells). Cinnamomum cassia (CCB: 100 µg/mL; used as positive control)。
The effects of inhibition of intracellular NF-κB activation after treatment with CM on AGS cells were examined by Western blotting (Fig. 5). To investigate the phosphorylation of NF-κΒ p65, cells pretreated with CM were infected with H. pylori at 50 : 1 infection ratio for 45 min. It is known that IκBα is phosphorylated in H. pylori-co-cultured cells30); however it is shown in our study, that IκBα degradation was inhibited by pretreatment of CM in concentration dependent manner. Likewise, H. pylori-induced NF-κB p65 phosphorylation was also inhibited by CM pretreatment (Figs. 5a, b).
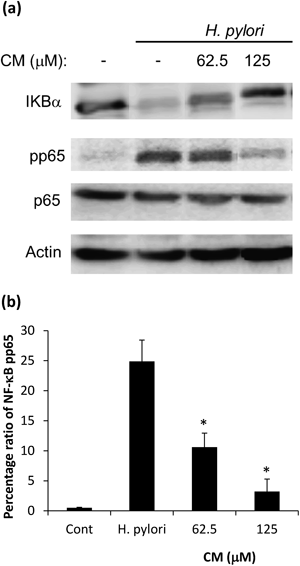
AGS cells were pretreated with CM at the indicated concentrations for 30 min followed by infection with H. pylori for 45 min. NF-κB phosphorylation and IκBα degradation in the cells were evaluated by immunoblotting using antibodies against NF-κB phospho-p65 (p-p65), NF-κB p65 (p65) and IκBα (Fig. 5a). Quantitation analysis of NF-κB pp65 inhibition is calculated (Fig. 5b). Actin is used as loading control.
Worldwide cancer reports estimate cancer as a leading cause of disease and deaths with an incidence of 12.7 million per year and mortality rate of 13% of all deaths.31,32) Out of all the new cancer cases diagnosed each year, stomach cancer accounted for 8% (990000) in the year 2008. These facts and figures ranked stomach cancer as the second most common cause of death from cancer worldwide.33) A wide geographical variation of gastric cancer incidence is seen, much of which is related to differences in diet and H. pylori infection. H. pylori infection plays a vital role in gastric diseases and, association between H. pylori infection and subsequent development of gastric cancer is reported as odds ratio (OR) of 2.36.34)
Recent advancement in cancer cell biology has shown probable mechanism behind H. pylori associated gastric pathogenesis. Firstly, the possibility of H. pylori stimulating inflammatory responses in gastric epithelial cells were identified,12) and severity of histologic gastritis in H. pylori-infected patients in correlation with mucosal IL-8 was noted.35) These inflammatory responses along with neutrophil infiltration ultimately lead to DNA damage apoptosis and carcinogenesis.36) H. pylori-associated gastric cancer, similar to other cancer, is a chronic process and symptoms might never appear until late stages.37) Hence, interest of researchers is now diverted to cancer chemoprevention in order to control pathogenic progress of cancer.20,23,30,38) One possible way to search for chemopreventive candidates is to search from traditional herbs and medicinal plants. Previously our group has identified C. cassia, out of twenty four traditionally used Pakistani medical plants, as potent anti-inflammatory candidate against H. pylori-induced inflammation in gastric epithelial cells.20) Previous studies confirm anti-inflammatory effect of C. cassia in non-infectious inflammation. Ranasinghe et al. have reviewed various studies demonstrating Cinnamon medicinal properties including in-vivo anti-ulceration effects in the stomach of rat in vivo.18) Also oral administration of Cinnamon extracts to mice significantly decreased LPS-induced inflammatory cytokine release from epithelial cells.39) However, to our knowledge, anti-inflammatory effects of C. cassia or CM have not been studied in H. pylori-induced gastric inflammation. In this study, we have first reported CM is a major anti-inflammatory constituent of C. cassia acting against H. pylori-induced inflammation of gastric cells.
Nowadays, a global surge is amplified to search for phytomedicine and traditionally used medicinal plants as anti-H. pylori agents. A recent review summarizes anti-H. pylori potentials of American, African, Brazilian, South and East Asian herbs.37) Previously a study has reported strong anti-H. pylori effect of distilled water extracts C. cassia at 100 mg/mL concentration,40) but our group showed little or no anti-H. pylori activity of ethanol extracts of C. cassia at lower concentration (≤500 µg/mL).27) Later we also confirm anti-inflammatory effect of C. cassia using five times lower than bactericidal concentration.20) However, controlled clinical trials of Cinnamon extracts had failed to eradicate H. pylori, and few other herbs were only able to improve H. pylori-associated symptoms.41) Hence, in our view, herbs such as C. cassia might have more potential as an anti-inflammatory rather than anti-H. pylori agent. In this study we report non-bactericidal concentration of CM (≤250 µM). This particular approach rationalizes anti-inflammatory effect of this drug at a non-bactericidal concentration. The similar drug concentrations were also confirmed to be non-cytotoxic for AGS and MKN-45 cells (Fig. 1).
Though, many culinary and medicinal plants are considered to possess anti-adhesive properties against H. pylori binding to gastric epithelial cells,26,40) CM did not show any such anti-adhesive property against H. pylori (Fig. 2). Therefore, the anti-inflammatory effect of CM observed in this study might not be due to inference of bacterial binding to the host cells. Our group had shown similar effects of resveratrol23) and curcumin,30) and this phenomenon was referred with the previously coined term of “chemoprevention.”20) Furthermore various other medicinal plants with similar properties are under investigation by our group and will be reported elsewhere.
Previously our results revealed strong IL-8 inhibitory activity of C. cassia in H. pylori-infected and TNF-α stimulated cells.20) This effect is further confirmed in this study along with IL8-inhibitory effects of CM, active constituent of C. cassia, by immunoassay and real-time PCR. A dose dependent inhibitory effect is seen on IL-8 secretion (Fig. 3) and expression (Fig. 4). A recent study42) has shown suppression of age-related inflammatory NF-κB activation by CM, therefore we speculated similar anti-inflammatory pathway to be involved in our study. Hence, we investigated inhibition of NF-κB activation in H. pylori infected gastric epithelial cells by CM.
H. pylori infection of gastric epithelial cells is known to induce activation of NF-κB via a signaling pathway involving IκB kinase α and β (IKKα/β).43) The NF-κB family of transcription factors consists of five members, out of which p65 is a positive regulator or gene expression. NF-κB is present in the cytoplasm and is bound to the inhibitory proteins (IκBα, IκBβ and IκBε). In response of suitable stimuli, IKKα/β phosphorylates IκBα (Ser-32 and Ser-36) leading to subsequent ubiquitination and degradation of IκBα. Following this degradation either protein kinase A (PKA) or IKKα/β phosphorylates p65 and subsequent nuclear translocation of NF-κB.44) In our results, CM shows inhibition of phosphorylation of p65 and prevention of IκBα degradation in a concentration dependent manner (Fig. 5).
Previous literatures have suggested CM may inhibit NF-κB activation. A study has shown that CM suppresses age related increase of the Rac1/p38 pathway, which down regulated NF-κB activity.42) Rac1 is known to modulate inflammation by coordinating the activity of pro-inflammatory NF-κB and antioxidant Nrf2 transcription factors. The C. cassia derived CM is also a potent activator of the Nrf2-orchestrated antioxidant response in human colon epithelial cells, which results in alleviation of NF-κB-mediated inflammation.45)
In conclusion, CM at concentration ≤125 µM plays a major role at suppressing the inflammation by down regulation of H. pylori-induced IL-8 expressions via inhibition of NF-κB activation in AGS cells. Hence these agents, after further in vivo and clinical studies, can be considered as a potential candidate for chemoprevention against various H. pylori related gastric pathogenic processes.
We are thankful to Prof. Dr. Hiroaki Sakurai (Dept. of Cancer Cell Biology, University of Toyama) for gifting primary antibodies for immunoblot analysis, Mrs. Toyomi Kozawa (Dept. of Gastroenterology, University of Toyama) for technical assistance and Mohammad Aziz Rajani (Dept. of Biological and Biomedical Sciences, Aga Khan University) for providing assistance in few experiments. This work was partially funded by Grant from the FHS-Research Committee of the Aga Khan University, Karachi, Pakistan.
The authors declare no conflict of interest.