2015 Volume 38 Issue 12 Pages 1975-1979
2015 Volume 38 Issue 12 Pages 1975-1979
Arachidonic acid (AA) is metabolized to epoxyeicosatrienoic acids (EETs) via cytochrome enzymes such as CYP 2C9, 2C8 and 2J2. EETs play a role in cardioprotection and regulation of blood pressure. Recently, adverse reactions such as sudden heart attack and fatal myocardial infarction were reported among patients taking angiotensin II receptor blockers (ARBs). As some ARBs have affinity for these CYP enzymes, metabolic inhibition of AA by ARBs is a possible cause for the increase in cardiovascular events. In this study, we quantitatively investigated the inhibitory effects of ARBs on the formation of EETs and further metabolites, dihydroxyeicosatrienoic acids (DHETs), from AA via CYP2C8. In incubations with recombinant CYP2C8 in vitro, the inhibitory effects were compared by measuring EETs and DHETs by HPLC-MS/MS. Inhibition of AA metabolism by ARBs was detected in a concentration-dependent manner with IC50 values of losartan (42.7 µM), telmisartan (49.5 µM), irbesartan (55.6 µM), olmesartan (66.2 µM), candesartan (108 µM), and valsartan (279 µM). Losartan, telmisartan and irbesartan, which reportedly accumulate in the liver and kidneys, have stronger inhibitory effects than other ARBs. The lower concentration of EETs leads to less protective action on the cardiovascular system and a higher incidence of adverse effects such as sudden heart attack and myocardial infarction in patients taking ARBs.
Arachidonic acid (AA), a component of the cell membrane, is known to be metabolized to prostaglandins, thromboxaneA2, leukotrienes and other bioactive substances1) (Fig. 1). AA is also metabolized to epoxyeicosatrienoic acids (EETs) via cytochrome P450 isoforms such as CYP 2C9, 2C8 and 2J2, and is further converted to the corresponding dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH).2–7) There are four structural isomers, 14,15-, 11,12-, 8,9- and 5,6-EET, that are reported to have vasodilatory and anti-inflammatory effects, while endothelium-derived hyperpolarizing factor (EDHF) action has been reported for 14,15-, 11,12-EET,8–12) preconditioning effects have been reported for 14,15-EET,13) and blood pressure regulating effects have been reported for 11,12-EET.14)

In this study, we focused on the CYP enzyme-mediated reactions.
Totah and Rettie reported that CYP2C8, which constitutes about 7% of total microsomal CYP content in the human liver, appears to be important for metabolizing AA.15) CYP2C8 mainly generates 14,15-, 11,12-EET from AA, and the ratio of 14,15-EET : 11,12-EET is reported to be 1.25 : 1.11) These results suggest that cardiovascular events such as hypertension and myocardial infarction are caused by decreased concentrations of these EETs.
Angiotensin II receptor blockers (ARBs) are used for hypertensive patients with diabetes because of their cardio- and renoprotective effects. However, effects of ARBs other than the lowering of hypertension have recently been reported.16) In a meta-analysis of large-scale clinical trials, Bangalore et al. found no detectable beneficial effects on the outcome of myocardial infarction or cardiovascular mortality, despite the lowering of blood pressure by ARBs when compared with placebo.17) In the present study, we investigated the inhibitory effects of ARBs on AA metabolism to EETs by CYP2C8.
14,15-, 11,12-, 8,9- and 5,6-EET, 14,15-, 11,12-, 8,9- and 5,6-DHET, and their corresponding deuterium-labeled materials (d11-EETs and d11-DHETs) except for d11-5,6-DHET (internal standards), and AA were purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.). Losartan potassium and telmisartan were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.), and candesartan, valsartan, irbesartan and olmesartan were purchased from AstaTech (Bristol, PA, U.S.A.). Acetonitrile, ethanol, methanol and ethyl acetate were of HPLC grade and purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Recombinant CYP2C8 (rCYP2C8), control microsomes and reduced nicotinamide adenine dinucleotide phosphate (NADPH) regeneration systems (solutions A, B) were purchased from Becton Dickinson (Franklin Lakes, NJ, U.S.A.). Baculovirus-infected insect cells that coexpressed CYP2C8 and cytochrome b5 were used for recombinant enzyme, and wild-type baculovirus-infected Hi5 microsomes were used as a control. Solid-phase extraction columns (OASIS® HLB) from Waters (Milford, MA, U.S.A.) were used. All other reagents were of HPLC or special grade.
EETs and DHETs, their corresponding d11-structures, recombinant enzymes and control microsomes were stored at −80°C. Stock solutions of ARBs were dissolved in methanol (1 mM), and were stored at −20°C. NADPH regeneration system solutions were also stored at −20°C. All stock solutions were dissolved immediately before use.
Incubation StudyEach ARB stock solution was evaporated and reconstituted to various final concentrations (0, 10, 50, 100, 500 µM) in incubation medium. Medium was added to 176 µL phosphate buffer (0.5 mM, pH 7.4), 10 µL of solution A and 2 µL of solution B (NADPH regeneration system), and 10 µL of rCYP2C8 or control microsomes containing 3.6 and 5.0 mg protein/mL, respectively, and was then pre-incubated for 3 min at 37°C. The incubation experiment was started by adding 2 µL of 2 mM AA (final concentration: 20 µM). After incubation for 30 min at 37°C, the reaction was stopped by adding 50 µL of ice-cold acetonitrile. The mixture was spiked with 50 µL of internal standard solution (final concentration: 0.1 µM of d11-8,9-DHET and 0.2 µM of other d11-EETs and d11-DHETs), and was diluted with 700 µL of water. A sample was mounted on 30-mg Oasis® HLB solid-phase extraction columns (pre-conditioned with 2 mL of methanol and 2 mL of water), eluted with 2 mL of ethyl acetate, and then evaporated under vacuum. The residue was then redissolved in 50 µL of 50% acetonitrile. The resultant solution was injected into the liquid chromatography/tandem mass spectrometry (LC-MS/MS) system. All incubations were carried out within the linear range of AA metabolism with respect to substance concentration, incubation time, and P450 content.
EETs and DHETs concentrations were determined by the method recently reported.18) An Agilent 1200 series (Santa Clara, CA, U.S.A.) LC system was used to separate samples. Separation was performed on an Ascentis Express C18 column measuring 100 mm ×2.1 mm with a particle size of 2.7 µm (Supelco, Sigma-Aldrich, St. Louis, MO, U.S.A.). The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Elution was performed at a flow rate of 0.3 mL/min with the following gradient; 50% B for 27 min, 50–90% B from 27 to 28 min, held 90% B from 28 to 35 min, and 8 min re-equilibration at 50% B from 35 to 43 min. Injection volume was 40 µL. The HPLC system was coupled to an API3200 Q Trap System (AB Sciex, Framingham, MA, U.S.A.) equipped with a turbo ion spray source. Electrospray ionization (ESI) was performed in negative ion mode with nebulizing, turbo spray and curtain gas, with optimal values set at 60, 70 and 10 psi, respectively. Turbo gas temperature was set at 700°C and ESI needle voltage was set at −4500 V. Multiple reaction monitoring (MRM) was employed using nitrogen as a collision gas, with a dwell time of 232 ms and 117 ms for EETs and DHETs at each transition. The m/z ratio of each compound was used according to the previous report.18) Data were acquired using Analyst® 1.4.2 software (Sciex). Quantitative reproducibility expressed as inter-day coefficient of variation (CV) was within 10.0%, except for 5,6-DHET (10.5%).
Data AnalysisIC50 was calculated by Sigma Plot® 12.5 (Systat Software, Inc., San Jose, CA, U.S.A.). Total eicosanoid production was calculated by the sum of the concentrations of EETs and DHETs.
StatisticsThe production rate of EETs was assessed by Dunnett’s test when no significant differences in distribution were observed by Bartlett’s test. When distribution was not equal, the production rate was assessed by the Steel method. The statistical software program R (ver. 2.14.2)19) was applied for further statistical evaluation. Results were considered significant at p<0.05.
The production rates of EETs incubated with ARBs are shown in Fig. 2. For 14,15-EET, the production rate decreased significantly after the addition of 50 µM losartan, telmisartan, olmesartan and irbesartan, and 500 µM candesartan and valsartan. For 11,12-EET, the production rate was significantly reduced by 10 µM irbesartan, and 50 µM losartan, telmisartan and olmesartan, and 500 µM candesartan and valsartan. Negligible amounts of 8,9-EET and 5,6-EET were produced by rCYP2C8. The formation rates of DHETs were 0.3–0.6 times lower compared to those of EETs. The IC50 values of losartan, telmisartan, irbesartan, olmesartan, candesartan and valsartan were calculated to be 42.7, 49.5, 55.6, 66.2, 108, and 279 µM, respectively.

ARBs are: (A) Losartan; (B) Telmisartan; (C) Irbesartan; (D) Olmesartan; (E) Candesartan; and (F) Valsartan. EETs are 14,15-EET (○), 11,12-EET (●). Almost all of 8,9- and 5,6-EET were not shown because of under the LOQ. The vertical axis shows eicosanoid production (pmol/pmol P450/min) via rCYP2C8, and the horizontal axis shows the concentration of each ARB. Data are expressed as mean±S.D. (n=3–4) * p<0.05; ** p<0.01, as compared with 0 µM ARB. ARB, angiotensin II receptor blocker; EET, eicosatrienoic acid; LOQ, limit of quantitation.
Figure 3 shows decreased production of total eicosanoids, including EETs and DHETs adding each ARB, compared to those without inhibitor. The decreased eicosanoid production by adding 50 µM valsartan and candesartan was significantly higher than with the addition of losartan, while the eicosanoid production with the addition of olmesartan, irbesartan and telmisartan was almost equal to that with the addition of losartan. The values with the addition of valsartan and candesartan were significantly higher, and the value with the addition of olmesartan tended to be greater than with the addition of 100 µM losartan. The value was significantly higher with the addition of 500 µM valsartan. The value with the addition of other ARBs, including candesartan, olmesartan, irbesartan and telmisartan was the same as that with the addition of 500 µM losartan.
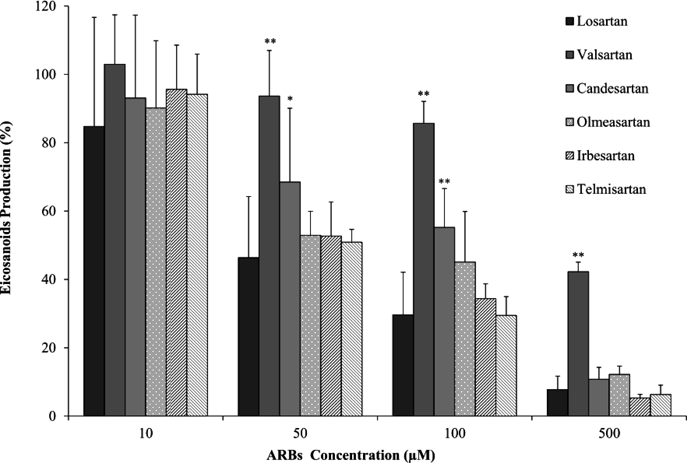
Data are expressed as mean+S.D. (n=3–4). ARBs are compared with each concentration of losartan (* p<0.05, ** p<0.01). ARB, angiotensin II receptor blocker.
Different inhibitory effects of ARBs on EETs production from AA via CYP2C8 were observed. The intensity of inhibitory effects was as follows by IC50; losartan>telmisartan>irbesartan>olmesartan>candesartan>valsartan. On auto-radiography, losartan, telmisartan and irbesartan were found to accumulate in the liver and kidneys rather than in the plasma.20,21) After administration of losartan to the rats, the concentration of ARBs was reported to be 30- and 2-fold higher in the liver and kidneys, respectively, when compared with plasma,20) while that in the liver was about 40-fold higher than in plasma after the administration of telmisartan.21) The IC50 values of CYP2C8 inhibition using amodiaquine as a substrate have been reported to be 12.9, 9.73, and 36.2 µM for losartan, irbesartan, and candesartan, respectively.22) These IC50 values were three to five times lower than our results, possibly because of using a different substrate. As these ARBs accumulated at 30 to 40-fold higher concentrations in the liver than in plasma, in which the total concentration range was reported to be 0.8–3.8 µM in patients taking ARBs,23) the ARB concentrations in this study were in the same range as in the liver in patients. Although high protein binding rates of ARBs have been reported in the systemic circulation,24) there is no information about the protein binding rates of ARBs in the liver.
Olmesartan, which has a lower affinity for CYP2C9,25) showed intermediate inhibition of CYP2C8 in the present study. Candesartan has been reported to have weak effects on the inhibition of CYP2C8, and to have negligible accumulation with rapid elimination of radioactivity in plasma after administration of 14C-labeled drugs.26) In the present study, valsartan, among the ARBs, showed less affinity to CYP enzymes. Theoretically, adverse cardiovascular effects caused by ARBs would be the result of CYP enzyme inhibition.27) Losartan, telmisartan and irbesartan have been found to show higher inhibitory effects on CYP metabolism and they are reported to accumulate in the liver and kidneys, resulting in decreased production of EETs and reduced physiological roles for cardiovascular tissues.26–28) The hyperpolarization of smooth muscles and vasodilation induced by EETs are mediated by activation of Ca2+-dependent K+ channels, with stronger cardioprotection leading to activation of K(ATP) channels.29) Considering that EET concentrations and the 14,15-EET/14,15-DHET ratio in plasma have negative correlations with the risk of cardiovascular events,30,31) a decrease of the EETs concentration in patients taking ARBs would correlate with an increase risk of cardiovascular events including sudden cardiac death and myocardial infarction.
EETs have an effect on cardioprotection including anti-inflammatory, vasodilating and myocardial preconditioning effects. The inhibitory effects of ARBs on CYP2C8 have been demonstrated to decrease the formation of EETs, which may theoretically increase the risk of cardiovascular events. Further clinical studies are necessary to investigate the inhibitory effects of ARBs on AA metabolism by human liver microsomes and to investigate the concentrations of EETs and DHETs in plasma under ARB administration, and their correlation with the frequency of cardiovascular events.
This work was supported by JSPS KAKENHI Grant Number 22590141. The authors would like to thank Ms. Mizuki Edasawa, Mr. Masatoshi Kando, and Mr. Shota Saiki for their technical assistance.
The authors declare no conflict of interest.