2015 Volume 38 Issue 2 Pages 325-330
2015 Volume 38 Issue 2 Pages 325-330
Four isoflavone-metabolizing bacteria were tested for their abilities to degrade (−)-epigallocatechin (EGC) and its isomer (−)-gallocatechin (GC). Biotransformation of both EGC and GC was observed with Adlercreutzia equolifaciens JCM 14793, Asaccharobacter celatus JCM 14811, and Slackia equolifaciens JCM 16059, but not Slackia isoflavoniconvertens JCM 16137. With respect to the degradation of EGC, strain JCM 14793 only catalyzed 4′-dehydroxylation to produce 4′-dehydroxylated EGC (7). Strain JCM 14811 mainly produced 7, along with a slight formation of the C ring-cleaving product 1-(3,4,5-trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1). Strain JCM 16059 catalyzed only C ring cleavage to form 1. Interestingly, the presence of hydrogen promoted the bioconversion of EGC by these bacteria. In addition, strain JCM 14811 revealed the ability to catalyze 4′-dehydroxylation of 1 to yield 1-(3,5-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2) in the presence of hydrogen. In the case of GC, strain JCM 14793 mainly produced C ring-cleaving product (1) with only a very small amount of 4′-dehydroxylated GC (8), while Strain JCM 14811 only catalyzed 4′-dehydroxylation to form 8. Strain JCM 16059 formed 1. The bioconversion of GC by the three strains was stimulated by hydrogen. Strain JCM 14793 showed the ability to convert 1 into 2 in the presence of hydrogen as did strain JCM 14811. Furthermore, strains JCM 14793 and JCM 14811 were found to have the ability to catalyze p-dehydroxylation of the pyrogallol moiety in the EGC metabolites 4-hydroxy-5-(3,4,5-trihydroxyphenyl)valeric acid (3) and 5-(3,4,5-trihydroxyphenyl)-γ-valerolactone (4), and this ability was enhanced by the presence of hydrogen.
(−)-Epigallocatechin gallate (EGCg) is well recognized as a major catechin in green tea, exhibiting various physiological functions including antioxidative, blood cholesterol lowering, hypoglycemic, and hypotensive activities.1) However, since absorption of intact EGCg in the body has been reported to be very low,2,3) it was considered to be important to elucidate its metabolic pathway in the gut tract in order to obtain further understanding of its physiological functions. We previously reported on the degradation of EGCg by rat intestinal microflora, identification of its metabolites, and its metabolic pathway in the gut tract.4) EGCg was found to be hydrolyzed to (−)-epigallocatechin (EGC) and gallic acid by intestinal bacteria at the first degradation step and then EGC underwent further degradation to produce various metabolites. We have recently reported on the isolation and characterization of rat intestinal bacteria involved in biotransformation of EGC.5) In this study, an isolate MT4s-5, named tentatively Adlercreutzia equolifaciens, was found to catalyze not only ring cleavage of (−)-epigallocatechin (EGC) to form 1-(3,4,5-trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1) but also 4′-dehydroxylation of both EGC and 1. The dehydroxylation reaction of 1 was enhanced by the presence of hydrogen, supplied by syntrophic bacteria such as Escherichia coli and Butyricimonas sp. strains. Ad. equolifaciens MT4s-5 also catalyzed p-dehydroxylation of 4-hydroxy-5-(3,4,5-trihydroxyphenyl)valeric acid (3) and 5-(3,4,5-trihydroxyphenyl)-γ-valerolactone (4) which were produced from 1 by Flavonifractor plautii MT42. Interestingly, Ad. equolifaciens MT4s-5 has been observed to convert daidzein to equol, and hence is considered to be responsible for the metabolism of isoflavone.6) The above phenomena led us to investigate whether or not intestinal bacteria involved in isoflavone metabolism possess degradation ability of EGC. Similarly, since bottled green tea contains considerable amounts of (−)-gallocatechin (GC) and (−)-gallocatechin gallate (GCg), the isomers of EGC and EGCg produced during sterilization process of the beverage, GC was also investigated.
In this report, we describe the abilities of several commercially available isoflavone-metabolizing bacteria7–12) to degrade EGC and its isomer GC. Furthermore, we note the p-dehydroxylation abilities of EGC metabolites, 3 and 4 by these bacteria.
(−)-Epigallocatechin (EGC) and (−)-gallocatechin (GC) were obtained from Sigma-Aldrich Japan (Tokyo). Metabolites, 1-(3,4,5-trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1), 1-(3,5-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2), 4-hydroxy-5-(3,4,5-trihydroxyphenyl)valeric acid (3), 5-(3,4,5-trihydroxyphenyl)-γ-valerolactone (4), 4-hydroxy-5-(3,5-dihydroxyphenyl)valeric acid (5), and 5-(3,5-dihydroxyphenyl)-γ-valerolactone (6) were prepared according to the methods previously reported4,5) and were used as reference standards. General anaerobic medium (GAM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Adlercreutzia equolifaciens MT4s-5 was isolated from rat feces as reported in our previous paper.5) Adlercreutzia equolifaciens JCM 14793,7) Asaccharobacter celatus JCM 14811,8,9) Slackia equolifaciens JCM 16059,10,11) and Slackia isoflavoniconvertens JCM 1613712) were purchased from Riken Bioresource Center (Ibaraki, Japan). All other chemicals were available products of analytical or HPLC grade. All cultures in this study were carried out under anaerobic condition with an AnaeroPack (anaerobic cultivation) system (Mitsubishi Gas Chemical, Tokyo, Japan) unless otherwise stated.
Analytical MethodsLC/MS analysis for structural identification was performed by a Surveyor HPLC and an LCQ Deca XPplus system (Thermo Fisher Scientific K. K., Yokohama, Japan) as described in our previous paper.4) LC/MS/MS analysis for quantitation was performed using a model Agilent 1100 series LC system (Agilent Technologies, Tokyo, Japan) coupled with a 3200QTRAP LC/MS/MS system (AB SCIEX, MA, U.S.A.) as reported in our previous paper.5) The optimized instrument setting of metabolite 8 was the same as that of metabolite 7 as mentioned in our previous paper.5) High-resolution FAB-MS (negative ionization mode) was carried out by a JEOL JMS-BU25 mass spectrometer using diethanolamine as a matrix. NMR analysis was carried out on a Bruker Ultrashield 400 plus system (1H, 400 MHz; 13C, 100 MHz: Bruker BioSpin K. K., Yokohama, Japan). All samples were dissolved in methanol-d4 (Kanto Chemical, Tokyo, Japan). Chemical shifts were referenced to tetramethylsilane (TMS) at 0 ppm. Specific rotations ([α]D20) of metabolites were measured by a P-1020 Polarimeter (JASCO Corporation, Tokyo, Japan).
Degradation of EGC and GC by Isoflavone-Metabolizing BacteriaFour bacterial strains, Ad. equolifaciens JCM 14793, As. celatus JCM 14811, S. equolifaciens JCM 16059 and S. isoflavoniconvertens JCM 16137, were separately precultured in GAM broth (5 mL) at 37°C for 48 h. Each preculture (0.5 mL) was then inoculated into 5 mL of fresh GAM broth containing 1 mM EGC or GC and was incubated at 37°C. GAM broth containing EGC or GC was also incubated without bacterium under the same condition as the control. After incubation for 24, 48, and 72 h, aliquots (0.5 mL) of the incubation mixture were withdrawn in an anaerobic glovebox under CO2 atmosphere. After adding 50 µL of 2 M HCl to each sample, bacterial cells were removed by centrifugation at 12000×g for 10 min at 4°C. A portion (0.2 mL) of each resulting supernatant was then diluted with 0.8 mL of 0.5% aqueous acetic acid, and the sample was analyzed by the LC/MS system for identification of metabolites and by the LC/MS/MS system for quantitation of metabolites as described in “Analytical Methods” section.
Degradation of EGC and GC by Isoflavone-Metabolizing Bacteria in the Presence of HydrogenEach preculture (0.5 mL) of the four strains was inoculated into GAM broth (5 mL) containing 1 mM EGC or GC and then hydrogen gas was aseptically bubbled into the incubation mixture for 10 s at a flow rate of about 50 mL/min. The resultant culture in test tube was packed in an Anaero Pouch (800 mL) with AnaeroPack system (Mitsubishi Gas Co., Inc.). Air in the pouch was roughly removed by hand and hydrogen gas was injected into the pouch for 30 s at a flow rate of 800 mL/min. Each of the resultant cultures was incubated anaerobically at 37°C. After sampling the cultures as described above, hydrogen gas was injected into the pouch again and the culture was continued. Samples for LC/MS and LC/MS/MS analyses were prepared in the same way as described above.
Dehydroxylation of EGC Metabolites 1, 3, and 4 by Isoflavone-Metabolizing BacteriaEach aqueous solution of 1, 3, and 4 was aseptically added to GAM broth (5 mL) to make the medium containing 1 mM of each of the metabolites. The medium was separately inoculated with each preculture of the four strains and was incubated anaerobically at 37°C. Samples for LC/MS and LC/MS/MS analyses were prepared in the same way as described before. Further, the above four strains were cultured in GAM broth each containing 1, 3, and 4 in the presence of hydrogen as the same conditions as described above.
Purification of Metabolites 7 and 8GAM broth preculture (10 mL) of Ad. equolifaciens JCM 14793 was poured into freshly prepared GAM broth (100 mL) containing 3 mM EGC and then hydrogen gas was aseptically bubbled into the incubation mixture. After filling up with hydrogen gas in an Anaero Pouch (800 mL) as described above, the resultant culture was incubated at 37°C for 48 h. After removal of bacterial cells by centrifugation (4500×g) for 15 min, the supernatant was extracted three times with equal volumes of ethyl acetate. The organic fraction was concentrated and metabolite 7 was purified by preparative HPLC as reported in our previous paper.4) Preparation of 8 was performed with As. celatus JCM 14811 and GC as substrate according to the same procedure as described above. Finally, purified 7 (76 mg) and 8 (68 mg) were obtained and were used as reference standards for quantitative analysis by LC/MS/MS system.
Metabolite 7: optical rotation [α]D20 −51.41° (c=0.49 in methanol). HR-MS m/z: 289.0721 [M−H]− (Calcd for C15H13O6 289.0718). 1H-NMR (methanol-d4) δ: 2.74 (1H, dd, J=16.8, 2.8 Hz, H-4b), 2.87 (1H, dd, J=16.8, 4.8 Hz, H-4a), 4.21 (1H, m, H-3), 4.81 (1H, s, H-2), 5.93 (1H, d, J=2.2 Hz, H-8), 5.94 (1H, d, J=2.2 Hz, H-6), 6.20 (1H, t, J=2.1 Hz, H-4′), 6.46 (2H, d, J=2.1 Hz, H-2′, 6′). 13C-NMR (methanol-d4) δ: 29.3 (C-4), 67.5 (C-3), 80.0 (C-2), 95.9 (C-8), 96.5 (C-6), 100.1 (C-4a), 102.7 (C-4′), 106.3 (C-2′, 6′), 143.0 (C-1′), 157.2 (C-8a), 157.8 (C-7), 158.1 (C-5), 159.4 (C-3′, 5′). This compound was presumed to be 4′-dehydrolylated EGC [(2S,3S)-flavan-3,3′,5,5′, 7-pentol].
Metabolite 8: optical rotation [α]D20 −13.95° (c=0.49 in methanol). HR-MS m/z: 289.0712 [M−H]− (Calcd for C15H13O6 289.0718). 1H-NMR (methanol-d4) δ: 2.52 (1H, dd, J=16.2, 7.4 Hz, H-4b), 2.76 (1H, dd, J=16.2, 5.0 Hz, H-4a), 4.00 (1H, m, H-3), 4.62 (1H, d, J=6.8 Hz, H-2), 5.88 (1H, d, J=2.4 Hz, H-8), 5.93 (1H, d, J=2.4 Hz, H-6), 6.20 (1H, t, J=2.1 Hz, H-4′), 6.33 (2H, d, J=2.1 Hz, H-2′, 6′). 13C-NMR (methanol-d4) δ: 27.8 (C-4), 68.7 (C-3), 82.7 (C-2), 95.6 (C-8), 96.4 (C-6), 100.7 (C-4a), 103.1 (C-4′), 106.5 (C-2′, 6′), 143.2 (C-1′), 156.7 (C-8a), 157.7 (C-5), 157.9 (C-7), 159.6 (C-3′, 5′). This compound was presumed to be 4′-dehydrolylated GC [(2R,3S)-flavan-3,3′,5,5′,7-pentol].
We first examined whether or not four isoflavone-metabolizing bacterial strains possessed degradation ability on EGC and/or GC. It was found that Adlercreutzia equolifaciens JCM 14793, Asaccharobacter celatus JCM 14811, and Slackia equolifaciens JCM 16059 catalyzed the biotransformation of both EGC and GC, but Slackia isoflavoniconvertens JCM 16137 did not.
In Ad. equolifaciens JCM 14793, EGC was converted into 4′-dehydroxylated EGC (7) and this conversion was markedly stimulated in the presence of hydrogen (Fig. 1). On the other hand, GC mainly underwent the C ring cleavage to form 1-(3,4,5-trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1), along with a very small amount of 4′-dehydroxylated GC (8) as shown in Fig. 2. The presence of hydrogen stimulated notably the formation of 1 and 8. Further, strain JCM 14793 expressed the ability to catalyze the 4′-dehydroxylation of 1 to form 1-(3,5-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2) in the presence of hydrogen (Fig. 2).

EGC (○), metabolites 1 (□), 2 (■), and 7 (◊).
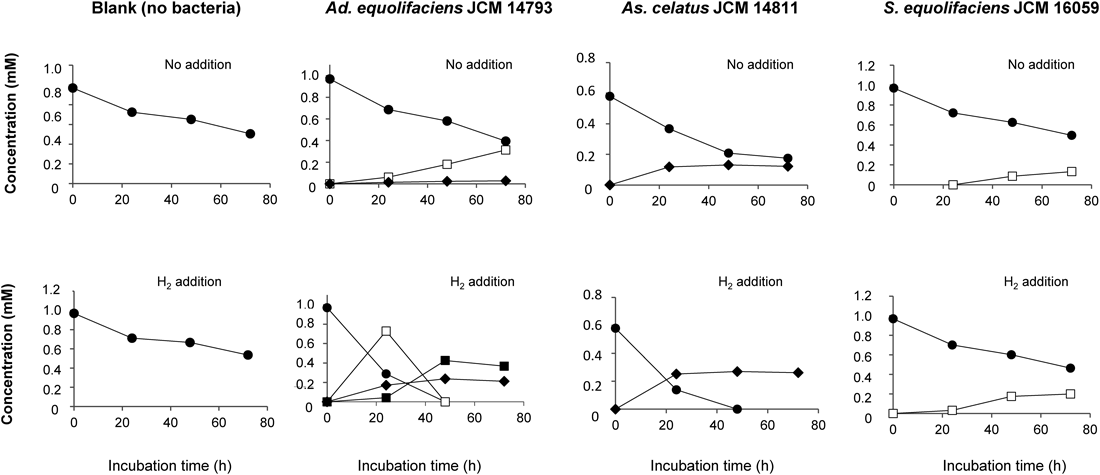
GC (●), metabolites 1 (□), 2 (■), and 8 (♦).
As. celatus JCM 14811 mainly produced 7 with a minor amount of 1 from EGC (Fig. 1). In the presence of hydrogen, strain JCM 14811 showed the conversion of 1 into 2 and the proportion of the C ring-cleaving product increased as compared with that produced without hydrogen (Fig. 1). With respect to GC, strain JCM 14811 produced only 8 and its production was stimulated by hydrogen (Fig. 2).
S. equolifaciens JCM 16059 catalyzed only the C ring-cleaving reaction of both EGC and GC to form 1 regardless of whether or not hydrogen was present (Figs. 1, 2). The reaction by this strain was found to be very slow but was stimulated to some degree by hydrogen (Figs. 1, 2). Interestingly, unlike strains JCM 14793 and JCM 14811, strain JCM 16059 did not show any ability to catalyze 4′-dehydroxylation of 1.
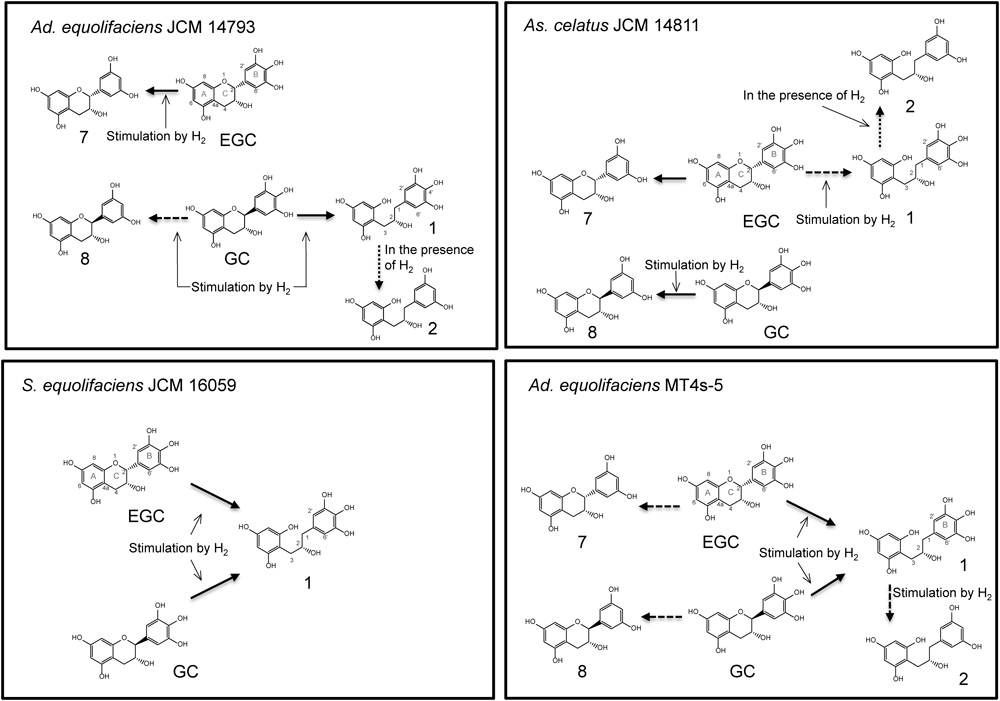
Ad. equolifaciens MT4s-5 has previously been reported to convert EGC to metabolites 1 and 7 together with a small amount of 2 and hydrogen facilitated the conversion of 1 into 2.5) However, since its ability to degrade GC was not then investigated, the bioconversion of GC was examined here. Strain MT4s-5 was observed to convert GC into 1 and 8 with slight production of 2. In the presence of hydrogen, strain MT4s-5 accelerated the formation of 2 (data not shown). The results were very similar to those obtained in the bioconversion of EGC by strain MT4s-5.5)
Dehydroxylation of Metabolites 1, 3, and 4 by Isoflavone-Metabolizing BacteriaWe further examined the dehydroxylation reaction of 1-(3,4,5-trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (1), 4-hydroxy-5-(3,4,5-trihydroxyphenyl)valeric acid (3), and 5-(3,4,5-trihydroxyphenyl)-γ-valerolactone (4) by the four isoflavone-metabolizing bacteria. Results showed that Ad. equolifaciens JCM 14793 and As. celatus JCM 14811 could catalyze p-dehydroxylation of the pyrogallol moiety in 1, 3, and 4, but S. equolifaciens JCM 16059 and S. isoflavoniconvertens JCM 16135 could not.
Ad. equolifaciens JCM 14793 converted 1 to 1-(3,5-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol (2) very slowly but this conversion was facilitated by hydrogen (Fig. 3). Strain JCM 14793 also caused p-dehydroxylation of 3 and 4 to produce 4-hydroxy-5-(3,5-dihydroxyphenyl)valeric acid (5) and 5-(3,5-dihydroxyphenyl)-γ-valerolactone (6), respectively, and the dehydroxylation was stimulated in the presence of hydrogen (Fig. 3).
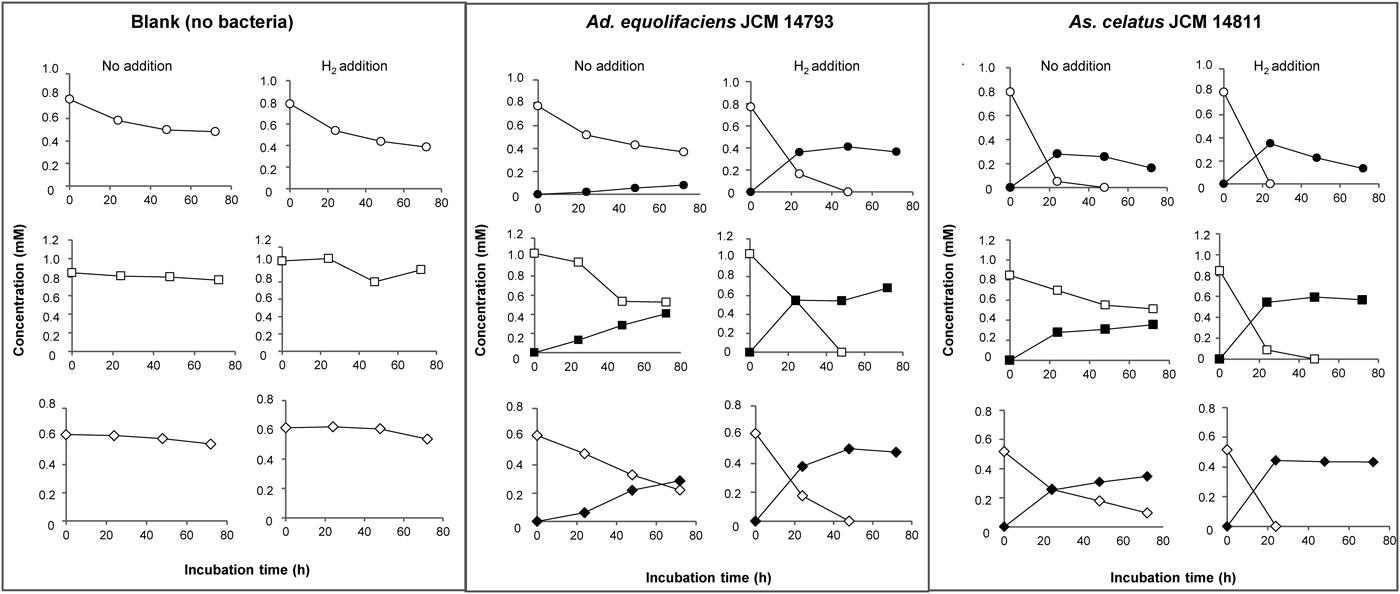
Metabolites 1 (○), 2 (●), 3 (□), 4 (◊), 5 (■), and 6 (♦).
As. celatus JCM 14811 could catalyze the dehydroxylation of 1 to form 2 and the dehydroxylation was somewhat accelerated by hydrogen (Fig. 3). Strain JCM 14811 also converted 3 and 4 into 5 and 6, respectively, and these conversions were enhanced in the presence of hydrogen, as they were also in the case of Ad. equolifaciens JCM 14793.
We have demonstrated here that several isoflavone-metabolizing bacteria possess the ability to biotransform EGC and GC. On the basis of these results, the behavior of the isoflavone-metabolizing bacteria on bioconversion of EGC and GC is illustrated in Fig. 4. In Ad. equolifaciens JCM 14793, EGC underwent 4′-dehydroxylation to form 7 whereas GC mainly underwent C ring cleavage to yield 1. Thus, strain JCM 14793 dramatically changed its catalytic reaction site from 4′-dehydroxylation to C ring cleavage, depending on the substrate configuration. As. celatus JCM 14811 catalyzed both 4′-dehydroxylation and C ring cleavage of EGC, whereas it catalyzed only the 4′-dehydroxylation of GC. Thus, the specificity of this strain’s reaction site was somewhat different for EGC and for GC. Furthermore, it was found that the reaction site specificity of Ad. equolifaciens JCM 14793 and As. celatus JCM 14811 varied to some extent in the presence of hydrogen. On the other hand, S. equolifaciens JCM 16059 and Ad. equolifaciens MT4s-5 showed no reaction site specificity differences between EGC and GC, either with or without hydrogen. Thus the above observations indicated differences in substrate and reaction site specificities existed among the microbial strains. Similar phenomena have been observed in the biotransformation of (+)-catechin and (−)-epicatechin by Eggerthella sp. strains SDG-2 and CAT-1.13)
Ad. equolifaciens JCM 14793 and As. celatus JCM 14811 were found to have the ability to catalyze p-dehydroxylation of 1, 3, and 4, as was Ad. equolifaciens MT4s-55) (Fig. 5). The p-dehydroxylation reaction was clearly stimulated in the presence of hydrogen. Although from the above observations Ad. equolifaciens JCM 14793, Ad. equolifaciens MT4s-5, and As. celatus JCM 14811 were expected to have the potential to catalyze p-dehydroxylation of gallic acid and pyrogallol which are produced from galloylated catechins (EGCg, GCg etc.) and from gallic acid, respectively, by intestinal bacteria,4,14,15) this was not the case (data not shown). In this connection, Wang et al.16) reported that Eggerthella sp. SDG-2 catalyzed p-dehydroxylation of 3,4-dihydroxyphenylpropionic acid to form 3-hydroxyphenylpropionic acid whereas this strain showed no dehydroxylation ability of 3,4-dihydroxyphenylacetic acid and gallic acid. They suggested that strain SDG-2 required at least three carbon atoms in the side chain for the dehydroxylation.
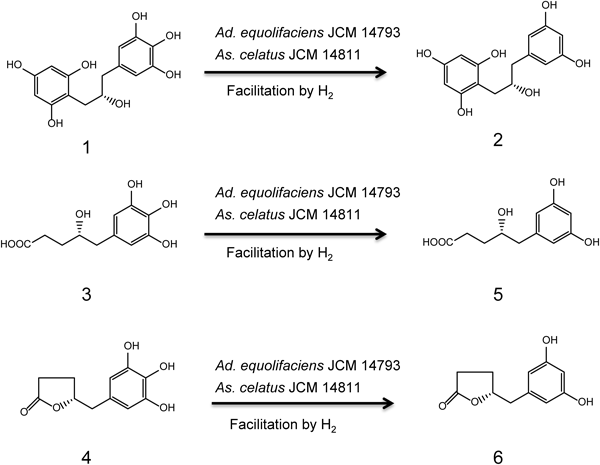
In this study, several isoflavone-metabolizing bacteria were shown to possess the ability to transform not only EGC and GC but also their metabolites (1, 3 and 4), and therefore may contribute to the metabolism of catechins in the intestinal tract.
We acknowledge the assistance of Andrea K. Suzuki (Mitsui Norin Co., Ltd.) in the proofreading of the manuscript.
The authors declare no conflict of interest.