2016 Volume 39 Issue 2 Pages 215-220
2016 Volume 39 Issue 2 Pages 215-220
Kupffer cells in livers bearing tumor metastases were found to have promoted tumor invasion and exacerbated the metastasis. This implies that the function of Kupffer cells might differ between animals bearing hepatic metastases and those that are healthy. Kupffer cells are considered responsible for the accumulation of liposomes in the liver. In this study, we hypothesized that the alteration in the function of Kupffer cells by hepatic metastasis would also affect the biodistribution of liposomes following intravenous administration. The hepatic accumulation and the blood concentration of PEGylated liposomes were compared between healthy mice and tumor-bearing mice. We noted that hepatic accumulation and elimination from the blood were significantly accelerated in tumor-bearing mice, indicating that our hypothesis was correct. In the tumor-bearing mice, the proportion of Kupffer cells taking up liposomes was significantly increased. Intravenous injection of oxaliplatin (l-OHP) containing PEGylated liposomes decreased the fraction of Kupffer cells, but this administration caused no injury to the hepatocytes. These results suggest that PEGylated liposomes containing l-OHP may have the potential to treat metastatic hepatic cancer—not only via the direct killing of the cancer cells but also via a reduction in tumor-supportive Kupffer cells.
The liver is known as a favored site for cancer metastasis due to its abundant blood flow. The general treatment for hepatic metastases is surgical resection. Chemotherapy is usually performed for preoperative treatment, preventing recurrence after surgery, or treating nonresectable tumors. However, a prescription regimen has not been well-established for hepatic metastases, particularly from noncolorectal primaries.1)
On the other hand, liposomal anticancer drugs have been developed. In particular, PEGylated liposomal anticancer drugs offer a high degree of stability in the blood and a low level of adverse effects. PEGylated liposomal doxorubicin (marketed as Doxil®/Caelyx®) has been approved for AIDS-related Kaposi’s sarcoma,2) ovarian cancer,3) and multiple myeloma.4) Moreover, clinical trials for the cancers of other organs are being actively conducted.5,6) From a viewpoint of biodistribution, it is notable that liposomes tend to accumulate in the liver.7) Therefore, treatments using liposomal anticancer drugs have been actively investigated in hepatocellular carcinomas.8,9) In particular, therapies combining the use of PEGylated liposomal doxorubicin and gemcitabine for advanced hepatocellular carcinoma have proceeded to phase II clinical trials.10) In metastatic hepatic cancer, Zhao et al. reported that a local perfusion of doxorubicin-loaded galactosylated liposomes is effective for liver metastasis from colorectal cancer.11)
Kupffer cells were thought to be responsible for the hepatic accumulation of liposomes.12,13) Kupffer cells phagocytose invasive pathogens and colloidal nanoparticles such as liposomes. They are also known to suppress the hepatic metastasis of tumors in the early stages.14,15) However, after a metastatic focus has formulated, Kupffer cells are known to help promote the invasion and further the metastasis of tumors.15) Therefore, the biological function of Kupffer cells seems to change in animals bearing metastatic tumors in the liver.
We hypothesized that if a metastatic tumor existing in the liver changes the biological function relating to the uptake of liposomes, then the pharmacokinetics and biodistribution of liposomes would also be changed. In the present study, liver metastasis increased the phagocytic activity of the Kupffer cells, but the liposomal anticancer drug, l-OHP-containing PEGylated liposome, decreased the fraction of Kupffer cells without severe liver damage.
Hydrogenated soy phosphatidylcholine (HSPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[methoxy(polyethylene glycol)-2000] (mPEG2000-DSPE) were generously donated by NOF (Tokyo, Japan). Cholesterol (CHOL) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Oxaliplatin (l-OHP) was generously donated by Taiho Pharmaceutical (Tokyo, Japan). 3,3′-Dioctadecyloxacarbocyanine perchlorate (DiO) was purchased from Invitrogen (Paisley, U.K.). All other reagents were of analytical grade.
AnimalsFemale mice, 5-week-old C57/BL6N and B6D2F1, were purchased from Japan SLC (Shizuoka, Japan). The mice had free access to water and mouse chow, and were housed under controlled environmental conditions (constant temperature, humidity and a 12 h dark/light cycle). All animal experiments were evaluated and approved by the Animal and Ethics Review Committee of Tokushima University. C57/BL6N mice were used for intraperitoneal tumor cell culture. The other animal experiments were performed using B6D2F1 mice.
Preparation of Metastatic Hepatic Cancer Model MiceM5076 ovarian sarcoma cells were kindly donated by Dr. Kazuki Nagasawa (Kyoto Pharmaceutical University). M5076 cells were cultured in the peritoneal cavity of C57BL/6N mice. The mice were euthanized and injected intraperitoneally with 5 mL of phosphate buffered saline (PBS). Cell suspensions were collected from the peritoneal cavity and centrifuged twice at 1700×g for 30 min at 20°C after adding 10 mL of distilled water. Cell pellets were resuspended in PBS. B6D2F1 mice were intravenously injected with cell suspensions (1×106 cells/100 µL PBS). All experiments using these mice were performed at day 16 after inoculation. This timepoint was chosen based on the finding by preliminary investigation that observable tumors would appear on the surface of the liver.
Preparation of Fluorescence-Labeled PEGylated Liposome and l-OHP-containing PEGylated LiposomesPEGylated liposomes composed of HSPC/CHOL/mPEG2000-DSPE (2/1/0.1, molar ratio) were prepared as follows. For cellular uptake experiments, 1 mol% of the fluorescent dye DiO was incorporated in the lipid mixture. The lipids were dissolved in chloroform, and after evaporation of the organic solvent, the resultant lipid film was hydrated in 10% sucrose. The liposomes were sized by subsequent extrusion through polycarbonate membrane filters (Whatman Inc., NJ, U.S.A.) with pore sizes of 400, 200, 100, and 80 nm. l-OHP-containing PEGylated liposomes were prepared as previously reported.16) The mean diameters of the prepared liposomes were determined using a NICOMP 380 ZLS (Particle Sizing System, CA, U.S.A.). The mean diameters for fluorescence-labeled PEGylated liposomes and l-OHP-containing PEGylated liposomes were 130±12 and 122±16 nm, respectively.
Biodistribution of l-OHP-Containing PEGylated LiposomesOn day 16 after tumor inoculation, mice were injected intravenously with l-OHP-containing PEGylated liposomes (4.2 mg l-OHP/kg). At 5 min, 1, 4, 8 or 24 h post-injection, blood was collected from the postcaval vein and the livers were excised. Plasma was separated by centrifugation. The livers were homogenized in 20 mL of PBS. Aliquots of the homogenates (1-mL) were lysed at 65°C with 0.3 mL of 30% hydrogen peroxide, and 1 M KOH (0.3-mL) in isopropanol. To the samples was added 10% acetic acid (0.3-mL). The samples were diluted to twice the volume for the liver and to 50-fold the volume for the blood using 1% Triton-X in 1 M HCl. The concentration of platinum in the sample was then quantified using an atomic absorption photometer (Z-5700, Hitachi, Tokyo, Japan). The concentration of l-OHP was calculated from the concentration of platinum using the molecular weight of platinum as an elementary substance and l-OHP. Elimination constant (kel) and hepatic clearance (CLh) were calculated using the bootstrap method, as previously described.17)
Evaluation of Liposome Uptake by Kupffer CellsOn day 16 after tumor inoculation, the mice were injected intravenously with DiO-labeled liposomes (25 mg HSPC/kg). Nontumorous healthy mice (healthy) were also treated in a similar way. The mice were euthanized at 24 h after administration and then laparotomized. The blood was removed from the livers by gently injecting Krebs–Ringer-Buffer (KRB) into the right ventricle. The livers were dissected and nonparenchymal cells were isolated following the recommended protocol for the cell isolation using gentleMACS dissociator supplied by Miltenyi Biotec K.K. In brief, the livers were washed in KRB. The livers were dispersed in Dissociation Mix (2 mM CaCl2, 2 mM MgCl2, 132 unit/mL Dnase I, 500 CDU/mL Collagenase IV) using a gentleMACS dissociator (Miltenyi Biotec, Germany). The liver suspensions were shaken at 37°C and 100 rpm for 20 min and resuspended using a gentleMACS dissociator. The liver suspensions were filtered using a cell strainer (100 µm; Becton Dickinson, NJ, U.S.A.) and subsequently added to PEB (0.5% bovine serum albumin (BSA), 2 mM ethylenediaminetetraacetic acid (EDTA) in PBS). Cell suspensions were centrifuged at 300×g and 4°C for 5 min, then resuspended in PEB. Hepatocytes were removed by centrifugation at 50×g and 4°C for 3 min. Nonparenchymal cells were obtained by subsequent centrifugation at 300×g and 4°C for 5 min. Nonparenchymal cell pellets were incubated for 2 min in 1 mL PEB and 10 mL hemolytic agent (154 mM NH4Cl, 10 mM KHCO3, 82 µM EDTA-4Na). The pellets were washed twice by centrifugation at 300×g and 4°C for 5 min in PEB. The number of total nonparenchymal cells was counted using a flow cytometer Guava EasyCyte™ Mini System (Guava Technologies, CA, U.S.A.).
The obtained nonparenchymal cells (5×105) were pelleted via centrifugation (300×g, 4°C, 5 min). The supernatant was removed and 100 µL of monoclonal antibody to Macrophage F4/80 antigen-PE (Acris Antibodies GmbH, CA, U.S.A.) in 0.5% BSA/PBS was added. The mixture was incubated at 4°C for 30 min and subsequently washed twice by centrifugation with 1 mL PBS. The cells were resuspended in 1 mL PBS and analyzed by flow cytometry using a Guava EasyCyte™ Mini System equipped with a 488 nm laser. Kupffer cells expressing F4/80 and the cells incorporating DiO-labeled liposomes were detected at emissions of 583 and 525 nm, respectively.
The number of total Kupffer cells was calculated from the number of total nonparenchymal cells the ratio of F4/80 positive cells calculated from the analysis result of the aliquot (5×105 cells) of nonparenchymal cells.
Effect of l-OHP Encapsulated in PEGylated Liposomes on the Number of Kupffer CellsOn day 16 after tumor inoculation, the mice were injected intravenously with l-OHP-containing PEGylated liposomes (4.2 mg l-OHP/kg). The mice were euthanized on either days 3, 5 or 7 after the administration of the liposomes. As a control, untreated mice were also euthanized on day 16 after the tumor inoculation. Nonparenchymal cells were obtained according to the method described above, and the amount of Kupffer cells was determined by flow cytometry.
Quantification of Serum TransaminaseHealthy mice were injected intravenously either with free l-OHP (4.2 mg/kg) or l-OHP-containing PEGylated liposomes (4.2 mg l-OHP/kg) on days 0, 3 and 6. Blood samples were collected at 24 h after the final administration of the liposomes. As a control, blood samples were also collected from the non-treated mice. The blood samples were centrifuged at 1700×g for 30 min at 4°C to obtain serum. The serum was then used for the quantification of serum aspartate aminotransferase (AST) and alanine transaminase (ALT) levels using a Wako Transaminase CII-Test kit (Wako Pure Chemical Industries, Ltd.).
Statistical AnalysisAll values were determined as the mean±standard deviation (S.D.). Statistical analysis was accomplished via a two-tailed unpaired Student’s t-test and one-way ANOVA followed by a Dunett post-hoc test using GraphPad InStat software (GraphPad Software, CA, U.S.A.). The level of significance was set at p<0.05.
The liver accumulation and plasma concentration of l-OHP following intravenous administration were compared between healthy mice and tumor-bearing mice (Fig. 1). The liver accumulation of l-OHP at 24 h after administration in tumor-bearing mice was significantly higher than that in healthy mice (Fig. 1A). The initial rate of liver uptake seemed to increase in the tumor-bearing mice. Concomitantly, the plasma concentration of l-OHP in tumor-bearing mice was significantly lower than that in healthy mice (Fig. 1B). The elimination rate constant of l-OHP in the tumor-bearing mice was increased 2.6-fold compared with that in healthy mice (Table 1), and there was a parallel 2.5-fold increase in hepatic clearance.
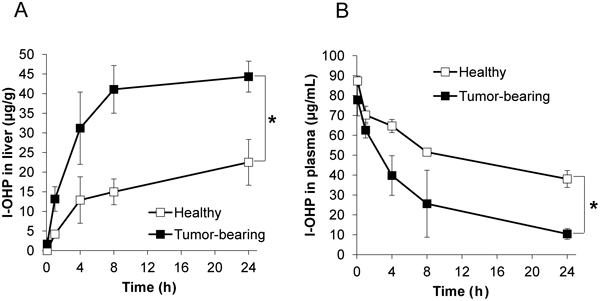
A) l-OHP accumulation in the liver. B) l-OHP concentration in the plasma. l-OHP-containing PEGylated liposomes were intravenously administrated into healthy mice and tumor-bearing mice. Mice were sacrificed and subjected to blood collection and liver dissection at 5 min, 1, 4, 8 or 24 h after administration. Platinum level in the sample was measured using an atomic absorption photometer. l-OHP concentration in the samples was calculated from platinum level. Data are represented as the mean±S.D. (* p<0.05).
| Healthy | Tumor-bearing | |
|---|---|---|
| kel (/h) | 3.05×10−2 | 7.96×10−2 |
| CLh (mL/min) | 3.94×10−3 | 9.71×10−3 |
To investigate the contribution of Kupffer cells to the increase in the liposomal l-OHP uptake in the liver, we evaluated the ratio of Kupffer cells, which took up PEGylated liposomes, to total Kupffer cells (Fig. 2). In tumor-bearing mice, the ratio of Kupffer cells taking up the PEGylated liposomes was significantly increased compared with that in normal healthy mice (Fig. 2A). This indicated that the increased accumulation of l-OHP-containing PEGylated liposomes in tumor-bearing mice (Fig. 1A) was due to the increased uptake of the liposomes by the Kupffer cells. Subsequently, the total number of Kupffer cells was determined (Fig. 2B). The number of Kupffer cells tended to increase in the tumor-bearing mice compared with that in healthy mice, although there was no significant difference.
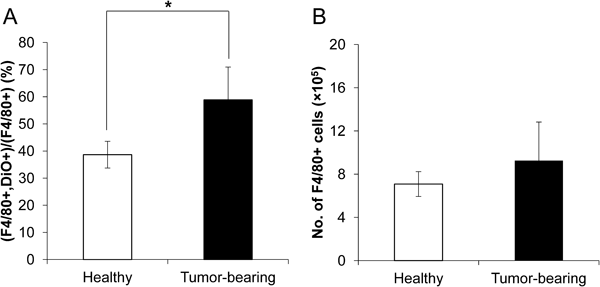
A) Ratio of Kupffer cells taking up DiO-labeled liposomes. B) Kupffer cells number of healthy mice and tumor bearing mice. DiO-labeled liposomes were intravenously administrated into healthy mice and tumor-bearing mice. Mice were sacrificed and subjected to liver dissection at 24 h after administration. Total Kupffer cells (F4/80+) and DiO-positive Kupffer cells were counted by flow cytometry. Data are represented as the mean±S.D. (* p<0.05).
The effect of the anticancer drug l-OHP when encapsulated in PEGylated liposomes on the number of Kupffer cells was investigated (Fig. 3). The proportion of Kupffer cells to nonparenchymal cells was maintained up to day 3 after administration, after which they started to decrease. The proportion was decreased to 70% at day 7, but they were not depleted.
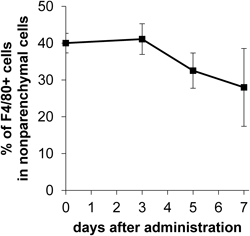
l-OHP-containing PEGylated liposomes (4.2 mg l-OHP/kg) were intravenously administrated into tumor-bearing mice. Mice were sacrificed and subjected to liver dissection at 3, 5, 7 d after administration. Total nonparenchymal cells and Kupffer cells (F4/80+) was counted by flow cytometer. The proportion of Kupffer cells was represented as the ratio of F4/80+ cell number to total nonparenchymal cell number. Data are represented as the mean±S.D.
In addition, the issue of whether l-OHP-containing PEGylated liposomes cause hepatotoxicity was studied. Healthy mice were used to investigate prevention of the influence of hepatic disorders that can be caused by liver metastases. Serum transaminases (AST and ALT) were measured in non-treated mice (control), in mice injected with l-OHP-containing PEGylated liposomes (liposomal l-OHP), and in mice injected with free l-OHP (Fig. 4). The mice receiving liposomal l-OHP showed significantly higher AST values than the control mice. However, the increased AST values observed in the mice that received liposomal l-OHP did not deviate from the standard values found in the literature,18) and there was no significant difference in the ALT values among all groups. These results suggested that liposomal l-OHP does not induce hepatotoxicity.
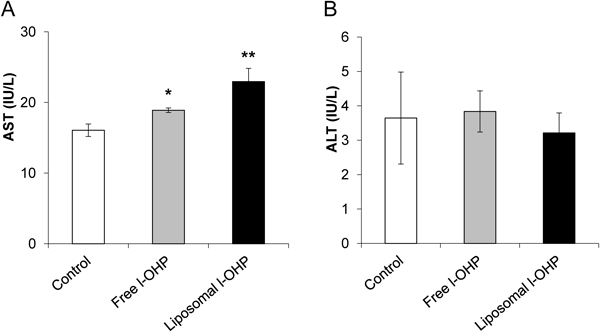
Free l-OHP (4.2 mg/kg) or l-OHP-containing PEGylated liposomes (4.2 mg l-OHP/kg) was intravenously administrated into healthy mice. Blood was collected at 24 h after administration. Serum AST and ALT were measured using Wako Transaminase CII-Test kit according to manufacturer’s protocol. Data are represented as the mean±S.D. (* p<0.05, ** p<0.01 vs. control).
In the present study, we demonstrated that the livers in mice bearing liver metastasis showed an increased uptake of PEGylated liposomes, resulting in a rapid elimination of PEGylated liposomes from the blood (Fig. 1, Table 1). The hepatic clearance of PEGylated liposomes in tumor-bearing mice was increased compared with that in normal mice (Table 1). This clearly suggests that the increase in the hepatic uptake of PEGylated liposomes was responsible for their rapid elimination from blood circulation of mice bearing a liver metastasis. Generally, the liver is considered to be the major organ for eliminating liposomes from the blood,7) and the primary cells that eliminate liposomes by endocytosis/phagocytosis are the Kupffer. Therefore, in the present study, the increased uptake of liposomes by the Kupffer cells was believed to be responsible for the increased liver accumulation of liposomes.
In the mice with liver metastasis, the ratio of Kupffer cells taking up PEGylated liposomes was increased (Fig. 2A). Intriguingly, this increase was only 1.5-fold, in contrast, the hepatic clearance was increased by 2.5-fold. This difference may be accounted for by accumulation to tumor tissue via enhanced permeability and retention (EPR) effect as a result of creating angiogenic vessels due to growing tumor cells. The angiogenic vessels in tumor were more permeable than normal vessels,19) and liposomes accumulate into tumor tissue via such angiogenic vessels.20) In addition, the total number of Kupffer cells tended to increase, but it was not significant in tumor-bearing mice (Fig. 2B). On the other hand, Kupffer cells are known to increase in hepatic metastasis model rats that are inoculated with CC531 carcinoma cells.21) The grade of cancer may account for the differences in Kupffer cell proliferation. In hepatocellular carcinoma, a tumor size-dependent decrease in the Kupffer cell number has been reported.22) Although only a few studies have focused on how the number of Kupffer cells varies with tumor progression in metastatic hepatic tumors, peritumoral Kupffer cell number has been correlated with prognosis.23) Therefore, the Kupffer cell number should also have relevance to the severity of metastatic tumors. Moreover, Kupffer cells often proliferate when the liver is in a pathological condition such as fibrosis.24) Restoration from hepatic damage also causes a proliferation of Kupffer cells.25,26) This evidence implies that not only tumor metastasis itself but also hepatic injury affects the increase in Kupffer cells. We assumed the following 2 mechanisms would account for the increased uptake of PEGylated liposomes by Kupffer cells in mice with liver metastasis. First, Kupffer cells are activated in mice with liver metastasis, resulting in increased endocytic/phagocytic activity of Kupffer cells. Consequently, Kupffer cells might further phagocytose/endocytose PEGylated liposomes. Natural killer (NK) cells are supposed to recognize migrated tumor cells and produce granulocyte macrophage colony-stimulating factor (GM-CSF) and interferon (IFN)-gamma, which enhances the killing activity of Kupffer cells against invasive pathogens.27) Kupffer cells, activated by NK cells, also are known to phagocytose more tumor cells.28,29) Therefore, in the current study, the activated Kupffer cells also contributed to the phagocytosis/endocytosis of PEGylated liposomes. The second mechanism concerns the alteration of the intrahepatic distribution of Kupffer cells in tumor-bearing mice. Angiogenic blood vessels existing in solid tumors show an increased permeability to nanoparticles within a diameter of 100 nm.30,31) Therefore, the nanocarriers passively accumulate in the solid tumor region,32) and Kupffer cells that accumulate in the tumor site gain opportunities to phagocytose/endocytose liposomes. Kan et al. reported on Kupffer cell distribution in the presence of metastatic tumors.29) They reported that Kupffer cells were attracted to metastatic tumor cells, adhering to the sinusoidal wall. However, Kupffer cells were significantly reduced in solid tumors, whereas they were significantly increased in Nontumorous sites. Based on those results, the accumulation of liposomes in a solid tumor might not result from the enhancement of Kupffer cell uptake. However, Kupffer cells outside a solid tumor can contribute to liposomal uptake via attraction by dispersed tumor cells in the sinusoid. We assumed that both activation and distribution of Kupffer cells should be involved in the enhancement of liposomal uptake by Kupffer cells.
The administration of l-OHP-containing PEGylated liposomes decreased the number of Kupffer cells from day 3 after administration (Fig. 3). In contrast, Doxil®, doxorubicin containing PEGylated liposome, was reported to have massively decreased the number of Kupffer cells by day 2 after administration.33) l-OHP is designed as an inhibitor of DNA synthesis in dividing cells such as tumor cells. Meanwhile, because the proliferation of Kupffer cells was insignificant under our experimental conditions, the mechanism of cell death for Kupffer cells seemed an intriguing matter. The toxicity of l-OHP in non-dividing cells has been well established in neuronal cells, because neurotoxicity is one of the major adverse effects of l-OHP.34,35) The dysfunction of ion channels and apoptosis caused by a dysfunction of the mitochondria are considered to be possible etiologies for neurotoxicity.36) Podratz et al. reported that cisplatin, a derivative of l-OHP, inhibited mtDNA replication and induced mitochondrial degradation in vitro and in vivo.37) As such, the mechanism for mitochondrial toxicity appears not to be specific to neuronal cells, and could also be a cause of toxicity in Kupffer cells. In addition, they also reported that mitochondrial degradation was increased in a time-dependent manner up to 6 d after the cisplatin treatment of cultured neurons.37) This seemed to support the hypothesis that Kupffer cell death by l-OHP occurred via mitochondrial degradation.
Of note, in this study, hepatotoxicity was not observed in the administration of l-OHP-containing PEGylated liposomes (4.2 mg l-OHP/kg) despite a significant reduction in Kupffer cell number (Fig. 4). This implies that hepatocytes were scarcely damaged by liposomal l-OHP. This may be explained by the differences between hepatocytes and Kupffer cells when interacting with l-OHP-containing PEGylated liposomes. To interact with hepatocytes, liposomes must cross the hepatic fenestration. Only smaller-sized liposomes (< 80 nm) have proven to be accessible to hepatocytes.38) Therefore, it might be difficult for our liposomal l-OHP (130 nm) to access hepatocytes. Such selective cytotoxicity of our liposomal l-OHP may make them applicable for the treatment of metastatic hepatic cancer for the purpose of reducing Kupffer cells without injuring hepatocytes. As mentioned earlier, Kupffer cells act as accomplices of tumors that have metastasized to the liver.15) Liposomal l-OHP may exert an antitumor effect by inducing cancer cell death and via reducing the ability of Kupffer cells to promote invasion and metastasis.
The authors are grateful to Mr. James L. McDonald for his helpful advice in developing the English manuscript. This study was supported by a research program for the development of intelligent Tokushima artificial exosome (iTEX) from Tokushima University.
The authors declare no conflict of interest.