2016 Volume 39 Issue 3 Pages 435-439
2016 Volume 39 Issue 3 Pages 435-439
A soluble form of human intestinal lactoferrin receptor (shLFR) is identical to human intelectin-1 (hITLN-1), a galactofuranose-binding protein that acts as a host defense against invading pathogenic microorganisms. We found that recombinant shLFR, expressed in mammalian cells (CHO DG44, COS-1, and RK13), binds tightly to Sepharose 4 Fast Flow (FF)-based matrices in a Ca2+-dependent manner. This binding of shLFR to Sepharose 4 FF-based matrices was inhibited by excess D-galactose, but not by D-glucose, suggesting that shLFR recognizes repeating units of α-1,6-linked D-galactose in Sepharose 4 FF. Furthermore, shLFR could bind to both Sepharose 4B- and Sepharose 6B-based matrices that were not crosslinked in a similar manner as to Sepharose 4 FF-based matrices. Therefore, shLFR (hITLN-1) binds to Sepharose-based matrices in a Ca2+-dependent manner. This binding property is most likely related to the ability, as host defense lectins, to recognize sepharose (agarobiose)-like structures present on the surface of invading pathogenic microorganisms.
Human intestinal lactoferrin receptor (hLFR) is a major pathway by which lactoferrin is taken up by enterocytes.1) A soluble form of hLFR (shLFR) is identical to human intelectin-1 (hITLN-1),2) a galactofuranose-binding protein that plays a role in host defense as lectins to assist in the phagocytic removal of microorganisms.3) hLFR is expressed as a glycosylphosphatidylinositol (GPI)-anchored protein on human intestinal epithelial cells, Caco-2,4) while hITLN-1 is expressed as a secretory protein (not as a GPI-anchored protein) in rabbit kidney epithelial cell (RK13) transfectants, despite being present on the cell surface.3) Although cell-specific expression of hLFR has been reported,3–5) a major form of hLFR (hITLN-1) was expressed as a secretory protein in insect4) and RK136) transfectants. Recombinant shLFR (hITLN-1) is a disulfide-linked, 120-kDa, homotrimeric polypeptide under nonreducing conditions, but is a 40-kDa, monomeric polypeptide under reducing conditions.4,7) Interactions between shLFR and human lactoferrin (hLF) are partially Ca2+ dependent, with an apparent Kd of 360 nM.4) hITLN-1 is considered a new type lectin that recognizes galactofuranose and binds to various mono/disaccharides, including D-galactose, in the presence of Ca2+.6) Because galactofuranose residues are present in the cell walls of various microorganisms but not in mammals, this suggests that hITLN-1 is a host defense lectin against invading pathogenic microorganisms.6) In fact, it has been reported that hITLN-1 recognizes the bacterial arabinogalactan of Nocardia, which contains D-galactofuranosyl residues, in a Ca2+ dependent manner.6) Additionally, hITLN-1 binds to Mycobacterium bovis bacillus Calmette–Guérin (BCG) and assists in the phagocytic removal of this microorganism.3)
In the present study, we found that recombinant shLFR (hITLN-1) binds tightly to Sepharose (4 Fast Flow [FF], 4B and 6B)-based matrices in a Ca2+-dependent manner. This binding property is most likely related to the ability of shLFR, as a host defense lectin, to recognize sepharose (agarobiose)-like, bacteria-specific components that are present on the surface of invading pathogenic microorganisms.
The reagents and media for cell cultures were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), except where indicated. The reagents for molecular biological experiments were obtained from Toyobo (Osaka, Japan), except where indicated. Epoxy-activated Sepharose 6B, Glutathione Sepharose 4B, Amersham Hybond 0.45 µm polyvinylidene difluoride (PVDF) and the Sepharose 4 FF-based matrices, Protein G Mag Sepharose, N-Hydroxysuccinimide (NHS) Mag Sepharose, and NHS-activated Sepharose 4 FF, were obtained from GE Healthcare UK Ltd. (Buckinghamshire, U.K.). Recombinant streptococcal protein G (P.4689) was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Cell CultureThe monkey kidney COS-1 cell line JCRB9082 was obtained from the Health Science Research Resource Bank (Osaka, Japan) and cultured in minimal essential medium (MEM)-α containing 10% heat-inactivated fetal bovine serum (FBS). The rabbit kidney RK13 cell line, RBRC-RCB0183, was provided by the RIKEN BRC (Ibaraki, Japan) and cultured in Eagle’s minimum essential medium (E-MEM) with 10% FBS and 1% non-essential amino acids. The CHO DG44 cell line (dhfr-) was obtained from Invitrogen (Carlsbad, CA, U.S.A.) and cultured in CD DG44 Medium (Invitrogen). The cells were maintained in 5% CO2 at 37°C.
cDNA Cloning of hLFRThe coding sequence for hLFR (identical to cDNA sequence of human intelectin-1 [GenBank™ accession No. AB036706]) was amplified by polymerase chain reaction (PCR) from a human prostate cDNA library (human prostate Marathon-Ready cDNA, Clontech Laboratories, Inc., Mountain View, CA, U.S.A.) using the following primers: LFRS, 5′-CTCGAGATG AAC CAA CTC AGC TTC CTG-3′; and LFRF-A, 5′-GCGGCCGCTCA ACG ATA GAA TAG AAG CAC A-3′.
The PCR product was cloned as an XhoI–NotI (underlined in the sequences above) fragment into mammalian cell expression vectors pCI-neo (Promega, Madison, WI, U.S.A.) (designated as pCI-neo LFRF) and pOptiVEC MCS8) (designated as pOptiVEC /LFRF). The sequences were verified before use.
Expression of shLFRThe shLFR expression vector (pCI-neo LFRF) was transiently transfected into COS-1 and RK13 cells using polyethylenimine Max (Polysciences Inc., Warrington, PA, U.S.A.). The transfected cells were incubated for 72 h at 37°C in Hybridoma-SFM (Invitrogen). The expression vector pOptiVEC/LFRF was transfected into CHO DG44 cells, and stable cell lines that expressed high levels shLFR were selected using the OptiCHO Express kit (Invitrogen), according to the manufacturer’s instructions as described previously.8) In cases where shLFR was expressed, stable transfectants were cultured in Hybridoma-SFM for 4 d at 37°C. Conditioned medium was centrifuged at 21500×g to remove cellular debris, and 10 µL of the supernatant was analyzed by Western blotting.
shLFR Binding AssayConditioned media from cultures of human intestinal lactoferrin receptor transfectants were used in binding assays because it has been reported that purified and concentrated shLFR is prone to aggregation in the presence of Ca2+.3,6,7) Fifty microliters of NHS-activated Sepharose 4 FF, Epoxy-activated Sepharose 6B blocked with ethanolamine or Glutathione Sepharose 4B were incubated with 300 µL of the culture supernatant from transfectants in the presence or absence of 5 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) at room temperature for 5h. In case of competitive binding with D-galactose or D-glucose, each monosaccharide at the indicated concentrations was added to the incubation step above. After washing five times with phosphate-buffered saline (PBS), bound shLFR was eluted from the Sepharose beads with 50 µL of Tris-buffered saline (TBS) containing 5 mM ethylenediaminetetraacetic acid (EDTA) (pH 8). Of note, the elution of bound shLFR at low pH (pH 2.5) was not adequate to obtain reproducible results, probably because concentrated shLFR is prone to aggregation in the presence of Ca2+.3,6,7) The eluate was centrifuged and filtered through 0.22 µm membrane filters to completely remove the Sepharose beads. The eluate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, followed by Western blotting (10 µL per lane was used in Figs. 2A, 3A, 4A, and 3 µL per lane was used in Figs. 2C, 3B, 4B).
Affinity Purification of shLFR Using NHS-Activated Sepharose 4 FFThree hundred fifty milliliter of conditioned medium from stable transfectants (CHO DG44 cell) was incubated overnight at 10°C with 1.4 mL of NHS-activated Sepharose 4 FF that had been blocked with ethanolamine and equilibrated with PBS. The Sepharose 4 FF suspension was collected into the empty column. After washing the column with 100 mL of PBS, bound shLFR was eluted with 1 mL of PBS containing 5 mM EDTA.
SDS-PAGE through a 10% Polyacrylamide Gel and Western Blotting AnalysesThe culture media of transfectants or the eluate in binding assays was subjected to SDS-PAGE through a 10% polyacrylamide gel. Samples were boiled (95°C for 3 min) prior to electrophoresis. Separated proteins were electroblotted onto a PVDF membrane, and the membrane was blocked with blocking buffer (Tris-buffered saline with 0.05% Tween-20 [TBST] and 1% bovine serum albumin (BSA)). The blot was probed with mouse anti-human Omentin 1 antibody (Omentin 1 [human]), mAb [Saly-1], Enzo Life Sciences, Farmingdale, NY, U.S.A.) and then the bound antibody was detected using alkaline phosphatase-conjugated anti-(mouse immunoglobulin G (IgG)), (Promega Corporation, Madison, WI, U.S.A.) as a secondary antibody. Color was developed using a commercial BCIP-NBT solution kit (Nacalai Tesque, Kyoto, Japan).
We have expressed the full cDNA sequence of hLFR in multiple mammalian cells (CHO DG44, COS-1, and RK13). As reported previously, hLFR was detected in the culture media as secreted soluble proteins in all tested mammalian cells. Western blot analysis indicated that shLFR was detected as a trimer of 120-kDa polypeptides under nonreducing conditions, and as a monomer of 40-kDa polypeptides under reducing conditions (Fig. 1), in accordance with previously published data.4,7) Faint bands of about 35- and 64-kDa under nonreducing conditions represented monomeric and dimeric forms, respectively. Three minor bands of >70-kDa under reducing conditions represented partially reduced shLFRs.
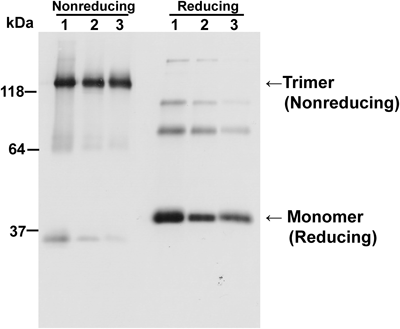
A shLFR was secreted into conditioned medium when the full cDNA sequence of hLFR was introduced into mammalian cells (CHO DG44, COS-1 and RK13). shLFR was detected as a 120-kDa trimer under nonreducing conditions, and as a 40-kDa monomer under reducing conditions. Lanes: 1, CHO DG44; 2, COS-1; 3, RK13.
In the binding analysis, we found that shLFR could bind to Sepharose 4 FF-based matrices, such as Protein G Mag Sepharose and NHS Mag Sepharose (data not shown). High concentrations (10 µM) of recombinant streptococcal protein G failed to block the binding of shLFR to Protein G Mag Sepharose, strongly suggesting that shLFR binds the Sepharose 4 FF-based matrices.
shLFR Binds NHS-Activated Sepharose 4 FF in a Ca2+-Dependent Manner and Most Likely Recognizes a Repeating Unit of α-1,6-Linked D-Galactose in Sepharose 4 FFWestern blot analysis confirmed that shLFRs in the culture media of transfectants (CHO DG44, COS-1, and RK13) bind NHS-activated Sepharose 4 FF blocked with ethanolamine (Fig. 2A, lane 2). This binding was abolished by the Ca2+ chelator, EGTA (Fig. 2A, lane 3). To further confirm the ability of shLFR to bind Sepharose 4 FF-based matrices, we attempted to purify shLFR in the conditioned medium of CHO DG44 stable transfectants using NHS-activated Sepharose 4 FF. shLFR absorbed to NHS-activated Sepharose 4 FF was successfully eluted with 5 mM EDTA. SDS-PAGE analysis, followed by Coomassie Brilliant Blue (CBB) staining indicated that the eluate is a trimer with a molecular weight of 120 kDa under nonreducing conditions, and is a monomer with a molecular weight of 40 kDa under reducing conditions (Fig. 2B), in agreement with previously published results.4,7) Thus, shLFR binds Sepharose 4 FF-based matrices in a Ca2+ dependent manner. We have obtained approximately 1.8 mg of purified shLFR from a 1L culture of CHO DG44 transfectants. Sepharose is an agarose-based polymer consisting of α-1,6-linked D-galactose and 3,6-anhydro-L-galactose (this unit is called agarobiose). shLFR was previously reported to be a lectin that recognizes D-galactose.6) Thus, we determined whether shLFR in the conditioned medium of CHO DG44 stable transfectants recognizes the repeating unit α-1,6-linked D-galactose in Sepharose 4 FF. Western blot analysis showed that interactions between shLFR and Sepharose 4 FF-based matrices were inhibited by D-galactose in a concentration dependent manner, but not by D-glucose (Fig. 2C). Therefore, shLFR appears to recognize repeating unit of α-1,6-linked D-galactose of agarobiose in Sepharose 4 FF-based matrices.
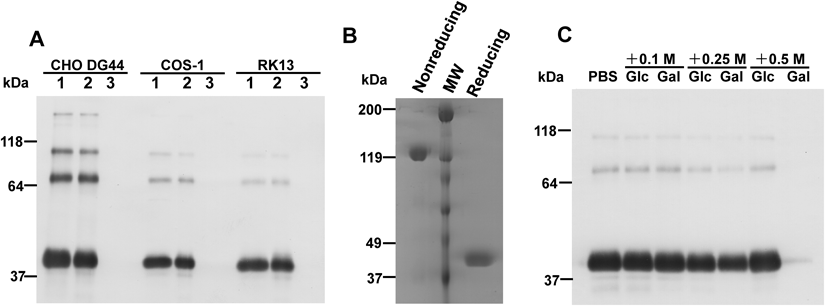
(A) Conditioned media from cultures of human intestinal lactoferrin receptor transfectants (CHO DG44, COS-1 and RK13) were subjected to a binding assay using NHS-activated Sepharose 4 FF, and the bound shLFR was analyzed by SDS-PAGE (under reducing conditions) and Western blotting. Lanes: 1, conditioned medium from each transfectant; 2, binding of shLFR to NHS-activated Sepharose 4 FF; 3, binding of shLFR to NHS-activated Sepharose 4 FF in the presence of 5 mM EGTA. (B) Affinity purification of shLFR with NHS-activated Sepharose 4 FF. Purified shLFR from the conditioned medium of CHO DG44 stable transfectants was subjected to 10% SDS-PAGE under nonreducing and reducing conditions. Coomassie Brilliant Blue staining is shown. MW: Molecular weight marker. (C) Inhibitory effects of D-galactose on the binding of shLFR to NHS-activated Sepharose 4 FF. Binding assay of shLFR in the conditioned medium of CHO DG44 stable transfectants with NHS-activated Sepharose 4 FF was carried out in the presence of D-galactose or D-glucose at the indicated concentrations. The bound shLFR was analyzed by SDS-PAGE (under reducing conditions) and Western blotting. PBS, binding of shLFR to NHS-activated Sepharose 4 FF. Glc, D-glucose; Gal, D-galactose.
Sepharose 4 FF is a crosslinked 4% agarose matrix. We next examined the influence of crosslinking and agarose concentration of Sepharose on the binding of shLFR to Sepharose matrices. Sepharose 4B- and 6B-based matrices were chosen because they are not cross-linked and contain 4 and 6% agarose, respectively. shLFR bound both Sepharose 4B- and 6B-based matrices in a Ca2+ dependent manner (Figs. 3A, 4A). Interactions of shLFR with non-crosslinked Sepharose were blocked by excess D-galactose, as shown in the binding analysis with Sepharose 4 FF (Figs. 3B, 4B), although their binding was also blocked with high concentrations of D-glucose. Sepharose 4B- and 6B-based matrices seemed to exhibit lower binding affinities to shLFR than Sepharose 4 FF-based matrices, because lower concentrations of D-galactose could block their binding. Therefore, crosslinking and agarose concentration of Sepharose did not influence the binding properties of shLFR to Sepharose matrices. shLFR most likely recognizes repeating unit of α-1,6-linked D-galactose of agarobiose in Sepharose-based matrices.

(A) shLFR binds to non-crosslinked Sepharose 4B-based matrices in the presence of Ca2+. Conditioned media from CHO DG44 stable transfectants were subjected to a binding assay using Glutathione Sepharose 4B and the bound shLFR was analyzed by SDS-PAGE (under reducing conditions) and Western blotting. 4 FF, binding of shLFR to NHS-activated Sepharose 4 FF; PBS, binding of shLFR to Glutathione Sepharose 4B; EGTA, binding of shLFR to Glutathione Sepharose 4B in the presence of 5 mM EGTA. (B) Inhibitory effects of D-galactose on the binding of shLFR to glutathione Sepharose 4B. Binding assay of shLFR in the conditioned medium of CHO DG44 stable transfectants with Glutathione Sepharose 4B was carried out in the presence of D-galactose or D-glucose at the indicated concentrations. The bound shLFR was analyzed by SDS-PAGE (under reducing conditions) and Western blotting. PBS, binding of shLFR to glutathione Sepharose 4B. Glc, D-glucose; Gal, D-galactose.
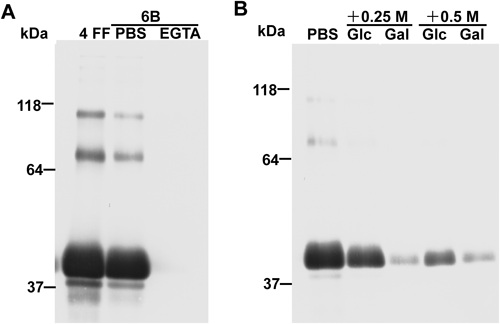
(A) shLFR binds to non-crosslinked Sepharose 6B-based matrices in the presence of Ca2+. Conditioned media from CHO DG44 stable transfectants were subjected to a binding assay using epoxy-activated Sepharose 6B blocked with ethanolamine and the bound shLFR was analyzed by SDS-PAGE (under reducing conditions) and Western blotting. 4 FF, binding of shLFR to NHS-activated Sepharose 4 FF; PBS, binding of shLFR to epoxy-activated Sepharose 6B; EGTA, binding of shLFR to epoxy-activated Sepharose 6B in the presence of 5 mM EGTA. (B) Inhibitory effects of D-galactose on the binding of shLFR to epoxy-activated Sepharose 6B. Binding assay of shLFR in the conditioned medium of CHO DG44 stable transfectants with epoxy-activated Sepharose 6B was carried out in the presence of D-galactose at the indicated concentrations. The bound shLFR was analyzed by SDS-PAGE (under reducing conditions) and Western blotting. PBS, binding of shLFR to epoxy-activated Sepharose 6B. Glc, D-glucose; Gal, D-galactose.
So far, four sepharose-binding lectins have been reported. Tachylectin-1 (formally called L6) of horseshoe crab binds Sepharose CL-6B in a Ca2+ dependent manner.9) Similarly, Hagfish C1q (HaC1q) binds to Sepharose 6B in a Ca2+ dependent manner.10) Interactions between fish-egg lectin (FEL) of the carp and Sepharose 4B were found to be partially Ca2+ dependent.11) Tachypleus plasma lectin (TPL-1) of horseshoe crab binds Sepharose CL-4B in a Ca2+ independent manner.12) shLFR did not show any significant sequence homology to the four sepharose-binding lectins described above.
Sepharose-binding lectins most likely act as a host defense mechanism by recognizing sepharose (agarobiose)-like structures present on the surface of invading pathogenic microorganisms.10) In fact, hITLN-1 (shLFR) was previously shown to recognize the bacterial arabinogalactan of Nocardia containing D-galactofuranosyl residues.6) It was also reported that hITLN-1 bound to Mycobacterium bovis bacillus Calmette–Guérin (BCG) and assisted in the phagocytic clearance of this microorganism.3)
In conclusion, shLFR (hITLN-1) binds to Sepharose-based matrices in a Ca2+-dependent manner, which is most likely related to the ability to recognize sepharose (agarobiose)-like structures present on the surface of invading pathogenic microorganisms.
The authors declare no conflict of interest.