2016 Volume 39 Issue 3 Pages 343-352
2016 Volume 39 Issue 3 Pages 343-352
Tacrolimus (TL) ointment is a topical treatment for atopic dermatitis, a disease that exhibits various skin conditions. The effect of skin pathologies on the systemic absorption of TL and related side effects remains unknown. This study aimed to investigate factors affecting the cutaneous absorption of TL. We prepared various skin models in hairless rats by tape stripping, injection of prophlogistic material solution (PMS), and continuous subcutaneous adrenaline (Adr) infusion. In vivo absorption studies were conducted, with measurements of transepidermal water loss (TEWL) and skin blood flow as physiological parameters. Very little TL absorption was observed through intact skin. Greater TL absorption was noted in skins with high TEWL values and fully stripped skin with PMS injections. In contrast, Adr infusion, which reduced skin blood flow, resulted in decreased TL absorption through fully stripped skin. Combined use of TL and Adr on skin with PMS injections resulted in suppression of TL absorption. Our results revealed that TL absorption following topical application is affected by alterations in the skin barrier, blood flow, and vascular permeability. We propose an administration plan for TL in a flowchart as a means of preventing systemic side effects.
Tacrolimus (TL, FK506, molecular weight of 803.5) is a 23-membered macrolide lactone isolated from the bacterium Streptomyces tsukubaensis. For over two decades, TL has been widely used as a calcineurin inhibitor immunosuppressant for organ transplantation, given intravenously and orally. A topical formulation of TL (Protopic® ointment, available in two TL ointment concentrations: 0.1% for adults and 0.03% for children) has also shown potent immunosuppressive activity and has been used as second line treatment for atopic dermatitis (AD) that cannot be controlled by topical corticosteroids.1,2) In 2006, the U.S. Food and Drug Administration (FDA) issued a black-box warning regarding a potential carcinogenic risk associated with TL ointment. Since then, some cohort studies and case-control studies have been conducted in order to reveal the association between topical use of TL and an increased risk of malignancy. Hui et al. and Arana et al. identified an increased risk of T-cell lymphoma with TL use.3,4) In contrast, other studies revealed no significant differences between TL use and non-use with regard to an increased risk of lymphoma.5,6) Due to short durations and potential study biases in these reports, results have been controversial. Thus, the FDA has advised that monitoring for occurrence of TL-related cancer be continued.
AD is a chronically relapsing inflammatory skin disease with severe pruritus and eczema. The skin barrier function in AD patients is disrupted and exhibits an increase in transepidermal water loss (TEWL).7–9) This skin disruption allows TL to permeate the skin despite a large molecular weight.10,11) TL exerts immunosuppressive effects on immunocompetent cells in the epidermis and dermis.12,13) The dermis exhibits altered conditions such as changes in blood flow, vascular hyperpermeability, and leakage of plasma components in AD patients.14–18) These alterations can change the migration of TL through the skin, which could affect TL absorption in the blood. However, it is still unclear as to how each alteration relates to TL absorption.
Formulations applied to the skin are classified into two types: transdermal drug delivery systems (TDDSs), which are expected to have systemic effects as a result of the distribution of the active substance through systemic circulation via blood capillaries in the dermis, and topical preparations, which exhibit local effects as a result of persisting in the skin or in the musculoskeletal system under the skin. In the case of TDDS, the formulation is generally applied on intact skin. Thus, the systemic absorption of TL has been studied primarily with intact skin, and not compromised skin.19) In vitro permeation studies have been performed using compromised skin and have shown alterations in the permeability of the skin.20) An in vitro study, however, is limited in its ability to reflect the influence of skin capillaries or complex physiological reactions, such as inflammation.
There have been few studies reported on the absorption properties of topically administered drugs in the systemic circulation through physiologically altered skin, and on the factors that influence these absorption properties. The aim of this study was to determine the relationship between systemic absorption of TL and physiological skin conditions, and furthermore, to provide an administration plan that is effective in improving therapeutic efficacy and avoiding side effects. As a preliminary study, we investigated the effects of skin barrier function and skin blood flow on the systemic absorption of TL following topical application on the dorsal skin in hairless rats. We created various physiological dorsal skin conditions: skin barrier disruption with repeated adhesive tape stripping, acute inflammatory reaction induced by intradermal injections of prophlogistic materials, and a hypovascular state induced by subcutaneous infusion of a vasoconstrictor, adrenaline (Adr). These physiologically altered skins were used for in vivo absorption studies, and conditions were simultaneously evaluated by measuring TEWL and skin blood flow as physiological parameters. Furthermore, we investigated whether topical application of Adr mixed with TL ointment prevented the systemic absorption of TL.
TL ointment (Protopic® ointment: 0.1 and 0.03%) was purchased from Astellas Pharma Inc. (Tokyo, Japan). Ascomycin was purchased from A.G. Scientific, Inc. (San Diego, CA, U.S.A.). Ethyl carbamate, liquid paraffin, Evans blue (EB), formic acid, λ-carrageenan, zymosan, and casein were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). L-Adrenaline was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Ammonium acetate was purchased from Sigma-Aldrich Inc. (Tokyo, Japan). Sodium carboxymethylcellulose (CMC Daicel 1170, 500–800 mPa·s, 25°C) was purchased from Daicel Chemical Industries Ltd. (Osaka, Japan). A microdialysis (MD) probe (OP-100-10) was purchased from Eicom (Kyoto, Japan). All other chemicals were of reagent grade quality.
AnimalsAnimal studies were performed according to the guidelines for animal use, approved by the Institutional Animal Care and Use Committee of Josai University (approval number: H27040—April 8, 2015). Male WBN/ILA-Ht hairless rats (Life Science Research Center, Josai University, Saitama, Japan) were housed under controlled temperatures, with a 12-h light/dark cycle, and were fed ad libitum until the in vivo experiments were conducted. The hairless rats (8–10 weeks old) were administered 5% isoflurane via inhalation for induction of anaesthesia, and were then anaesthetized using intraperitoneal injection of urethane (ethyl carbamate, 1.5 g/kg).
Preparation of Adr-Mixed TL OintmentThe Adr-mixed TL ointment (Adr, 0.25 and 0.50% (w/w)) was prepared with TL ointment and Adr, adequately mixed using a stainless steel spatula on a glass dish before use.
In Vivo Measurement of TEWLFor evaluating stratum corneum (SC) barrier function, TEWL measurements from the TL ointment application site were conducted using a VapoMeter SWL4001JT (Delfin Technologies, Kuopio, Finland), prior to administration of TL ointment in the in vivo absorption studies. Ambient conditions were controlled with a temperature of 25–26°C and 50–60% relative humidity during all experiments.
In Vivo Measurement of Skin Blood Flow with Laser Doppler FlowmetryWith respect to skin blood flow measurements, laser Doppler perfusion imaging scanning on the TL ointment application site was performed to calculate perfusion units (PU) using a PeriScan PIM 3 (Perimed AB, Stockholm, Sweden). This was conducted prior to administration of TL ointment, and immediately following the administration at time 0 min, concurrent with a schedule of blood sampling in the in vivo absorption studies.
Induction of an Acute Inflammatory Reaction in SkinA prophlogistic material solution (PMS); a mixed solution of λ-carrageenan (0.50%), zymosan (1.0%), and casein (1.0%) in 0.20% CMC saline solution was intracutaneously injected at 6 different sites on the TL application area, with an injection volume of 50 µL per site, to induce an inflammatory reaction.21) To confirm induction of inflammation in the skin, EB saline solution (1.0%) was intravenously injected (1.0 mL), with PMS intracutaneously injected 5 min following EB administration.
Induction of Vasoconstriction in Skin Capillaries with AdrAn MD probe with a semipermeable membrane (molecular weight cut off value: 50 kDa) was subcutaneously inserted using a 21 gauge-injection needle as a guide cannula. To alleviate skin tissue trauma caused by probe insertion, phosphate buffered saline (PBS, pH 7.4) was perfused at a rate of 1 µL/min, using a syringe pump, for a 60 min equilibration period.22,23) After the equilibration, the perfusion medium was changed to Adr solution (Adr in PBS, 500 µg/mL) and perfused for 60 min in order to obtain a steady-state skin blood flow prior to the in vivo absorption study.
In Vivo Skin TL Absorption StudyFollowing anaesthesia, the hair of the dorsal skin was removed using an electric razor. To remove SC, tape stripping with adhesive tape was conducted. Tape stripping was performed 5, 10, 15, or 20 times, in order to obtain various degrees of SC barrier function. The skin stripped 20 times was regarded as “fully stripped” skin in this study. The application site for TL was marked with a marker pen. TL ointment was applied under occlusive conditions in all experiments, i.e., application sites were wrapped with polyethylene films that were fixed with adhesive tape. TL ointment dosage was 10 mg/cm2. Rats were placed in a supine position briefly for collection of a blood sample from the jugular vein, before being returned to the prone position.
Sample PreparationTo 100 µL of blood sample, 150 µL of a mixed solution of methanol and 0.1 M zinc sulfate solution (7 : 3, v/v) was added and vortexed for 10 s. Subsequently, 250 µL of the internal standard solution (ascomycin in acetonitrile, 10 ng/mL) was added and vortexed for 30 s. Following centrifugation of this mixture at 14000 rpm for 5 min, 450 µL of the supernatant was evaporated at 45°C under vacuum (5.1 Torr) in a SpeedVac 2010 (Thermo Fisher Scientific Inc., Yokohama, Japan). The residue was redissolved in 70 µL of the mobile phase and centrifuged at 14000 rpm for 5 min. The supernatant (10 µL) obtained from the reconstituted solution was injected into a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system.
LC-MS/MS ConditionsTL blood concentration measurement was performed using LC-MS/MS analysis with a Prominence modular high-performance liquid chromatograph (Shimadzu, Kyoto, Japan) coupled to an API4000 (AB SCIEX, Tokyo, Japan). A Hypersil GOLD CN column (3 µm, 2.1×150 mm, Thermo Fisher Scientific Inc.) fitted with a Hypersil GOLD CN guard column (3 µm, 2.1×10 mm, Thermo Fisher Scientific Inc.) and a guard cartridge (2.1×4.6 mm, Thermo Fisher Scientific Inc.), was held at a temperature of 30°C. The mobile phase consisted of 0.1% formic acid and 2 mM ammonium acetate in a mixed solution of acetonitrile and water (65 : 35, v/v), and was isocratically eluted at a flow rate of 0.20 mL/min. The injection volume of each sample was 10 µL. Mass spectra were detected by performing electrospray ionization in a positive ion mode. For the MS/MS analysis, the parent ions and daughter ions were selected at 822.5 m/z ([M+NH3]+ ion) and 769.5 m/z for TL, and at 810.5 m/z ([M+NH3]+ ion) and 757.4 m/z for ascomycin, respectively.
Data AnalysisAll data is presented as mean values (±standard deviation (S.D.) where indicated). The area under the concentration versus time curve (AUCblood) of TL collected (0–8 h) was calculated using the trapezoid rule, as well as the area under the PU versus time curve (AUCPU). Statistical significance was calculated using Student’s t-tests and the Tukey–Kramer method for multiple independent comparisons. Correlation coefficients and normal p-values were calculated with the Pearson test. Differences were considered significant at p<0.05, 0.01, 0.001, and 0.0001. All statistical analyses were performed with GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, U.S.A.).
The 0.1% TL ointment was applied (10 cm2) on the dorsal rat skin with an intact SC barrier. Table 1 shows the TEWL and PU values measured before the topical application of TL on intact skin, and the TL concentrations in whole blood 15 min, 90 min, and 8 h after TL application. In intact skin without intracutaneous PMS injections [PMS (−)], the mean TEWL and PU values (±S.D.) were 6.7±0.8 g/m2/h and 151±33, respectively. Whole blood TL concentrations were very low in four of six rats, and were below the quantitation limit of 0.06 ng/mL in two of six rats, over the 8 h period of this study.
| PMS | Rat No. | TEWL (g/m2/h) | PU | TL whole blood concentration (ng/mL) | ||
|---|---|---|---|---|---|---|
| 15 min | 90 min | 8 h | ||||
| (−) | 1 | 6.1 | 130 | —* | — | 0.07 |
| 2 | 6.0 | 123 | — | — | — | |
| 3 | 6.1 | 166 | — | — | — | |
| 4 | 6.8 | 187 | — | 0.14 | 0.11 | |
| 5 | 7.9 | 114 | — | 0.12 | 0.12 | |
| 6 | 7.2 | 187 | — | 0.09 | — | |
| Mean±S.D. | 6.7±0.8 | 151±33 | ||||
| (+) | 1 | 5.7 | 137 | — | — | 0.07 |
| 2 | 5.2 | 134 | — | — | — | |
| 3 | 9.2 | 70 | — | — | — | |
| 4 | 6.3 | 161 | — | — | 0.06 | |
| 5 | 6.3 | 154 | — | 0.10 | 0.10 | |
| 6 | 7.6 | 222 | — | 0.13 | 0.13 | |
| Mean±S.D. | 6.7±1.5 | 146±49 | ||||
* —: Not detected, limit of quantitation: 0.06 ng/mL. PMS=prophlogistic material solution; TEWL=transepidermal water loss; PU=perfusion unit; TL=tacrolimus; S.D.=standard deviation.
The 0.1% TL ointment was applied (10 cm2) on the dorsal skin of rats intracutaneously injected with PMS [PMS (+)]. Before the in vivo absorption study, to confirm the induction of skin inflammation, EB saline solution (1.0%) was intravenously injected (1.0 mL), with PMS intracutaneously injected 5 min later. EB extravasation was observed in the skin excised 30 min after the PMS injection (data not shown), suggesting that the PMS injection induced vascular hyperpermeability and cutaneous oedema accompanied by plasma protein leakage into the skin tissue. In the skin intracutaneously injected with PMS, the in vivo absorption study showed the mean values of TEWL and PU (±S.D.) to be 6.7±1.5 g/m2/h and 146±49, respectively. On data analysis, there was no significant difference in the PU and TEWL values between skins with and without PMS injection. The whole blood TL concentrations were very low or undetectable in the oedematous skin. These results suggest that systemic absorption of TL through the skin with an intact SC barrier is rare, and inflammation under intact SC has no effect on TL absorption.
Effect of Varying Degrees of Skin Barrier Disruption on Systemic TL AbsorptionTL concentration in ointment and the application area should be taken into consideration when attempting to understand the relationship between the application condition of TL ointment and the behaviour of systemic absorption through the skin. TL concentrations in ointment (0.1, 0.03 and 0.003% (w/w)) and application areas (0.79, 5.0 and 10 cm2) were tested on fully stripped skin to remove the influence of individual difference in SC barrier function. The 0.003% TL ointment was prepared by diluting the 0.1% TL ointment with liquid paraffin. Figure 1A shows the TL concentration–time profiles under varied TL ointment concentration or application area. Both AUCblood and the parameters examined showed linear relationships (Figs. 1B, C), suggesting that the systemic absorption of TL through fully stripped skin follows diffusion theory.24)

Symbol form in (A), (B) and (C) represent TL concentration of ointment: open, 0.1%; grey, 0.03%; closed, 0.003%. Symbol shapes in (A), (B) and (C) represent application area of TL ointment: square, 10 cm2; circle, 5.0 cm2; triangle, 0.79 cm2. Each value is expressed as the mean±S.D. (n=3–6). TEWL measurement in (D) was taken in the skin treated with full stripping (open diamond) or controlled stripping (closed diamond) prior to TL application. The regression curve in (D): (AUCblood/TL dose=(3.9702×TEWL)−34.893) and (AUCblood/application area/TL concentration in the ointment applied)=(0.397×TEWL)−3.489). The r and p-values were calculated using the Pearson’s test.
In order to disrupt the SC barrier, and to achieve varying degrees of skin disruption, the number of tape strippings was varied, with the degree of barrier disruption estimated by measuring the TEWL. The TL ointment (0.1, 0.03 or 0.003% (w/w)) was applied (0.79, 5.0 or 10 cm2) on the skin treated with controlled tape stripping or full stripping. Figure 1D shows the relationship between TEWL values measured before TL application, and the AUCblood values over 8 h following TL application. Due to varied application areas and ointment concentrations, AUCblood is displayed as values divided by each TL dose, or each application area and ointment concentration. These values represent the varying degrees of barrier disruption in each rat. A highly significant, statistically positive correlation was observed between TEWL and AUCblood values. Pearson’s correlation coefficient (r) and the two-tailed p-value for this correlation were r=0.941 and p<0.0001. This result suggests that systemic absorption of TL through rat skin strongly depends on SC barrier function.
Effect of Intracutaneous Injection of PMS in Skin Lacking SC Barrier on the Systemic Absorption of TLIn order to investigate the effect of intracutaneous injection of PMS on the systemic absorption of TL through barrier-disrupted skin, the dorsal skin of rats was fully stripped and intracutaneously injected with PMS to induce acute inflammation under the TL application area. Thirty minutes after the PMS injections, the 0.1% TL ointment was applied (5.0 cm2) on the oedematous skin. Mean TEWL and PU values (±S.D.) were 252.3±17.1 g/m2/h and 347±46 in the fully stripped skin without PMS injections, and 234.7±19.5 g/m2/h and 326±99 in the fully stripped skin with PMS injections. Fully stripping the skin increased both the TEWL and PU values, irrespective of PMS injection status, suggesting that skin blood flow is affected by physical stimulus and that the increase in skin blood flow, enhanced by tape stripping, might contribute to TL absorption. Although no difference in the TEWL and PU values between skins with and without PMS injections was observed, significantly higher whole blood TL concentrations were observed in the oedematous skin, when compared to that without PMS injections (Fig. 2). This result indicates that there are other factors related to TL absorption in SC barrier-disrupted skin.

Symbols: closed circle, intradermal injection of the PMS; open circle, no PMS injection. Each value is expressed as the mean±S.D. (n=4, 11); * p<0.05, ** p<0.01 for each time point between two groups, calculated with Student’s t-tests.
Full tape stripping increased skin blood flow, while PMS injections exhibited no change in skin blood flow. In order to investigate the effect of skin blood flow on the systemic absorption of TL in the fully stripped skin, the continuous subcutaneous infusion of Adr solution was performed using an MD probe [Adr (+)]. For comparison, Adr-free PBS was infused in the fully stripped skin [Adr (−)]. Measuring skin blood flow prior to application of TL, it was observed that Adr infusion showed significantly lower PU values (p<0.01) when compared to the Adr-free PBS infusion (Fig. 3B). Adr infusion also showed significantly lower TEWL values (p<0.01) (Fig. 3A), which may be due to a decrease in skin blood flow. The 0.1% TL ointment was applied (0.79 cm2) on both Adr-infused and non-infused skin. Figure 3C shows the TL blood concentration–time profiles following TL application. TL concentrations were significantly lower in the Adr-infused skin than in the non-infused skin over the 2 h period of this study, suggesting that poor blood flow limits systemic absorption of TL in skin with a disrupted SC.

Symbols in (C): open circle, infusion of Adr-free PBS (infusion rate, 1 µL/min); open square, infusion of Adr solution (Adr in PBS, 500 µg/mL; infusion rate, 1 µL/min). Each value is expressed as the mean±S.D. (n=3, 4): (A, B), ** p<0.01, Student’s t-test; (C), ** p<0.01, *** p<0.001 for each time point between two groups, calculated with Student’s t-tests.
Considering the therapeutic aspects of TL ointment use, the combined topical use of TL and Adr was expected to prevent TL absorption into the bloodstream. We applied 0.1% TL ointment mixed with Adr on the fully stripped skin (5.0 cm2), with or without PMS injection. The TEWL and PU values prior to application of TL showed no significant differences among all five groups (Figs. 4A, B). In contrast, the AUCPU values that were calculated from the PU–time profiles (Fig. 4C) during the in vivo absorption study, showed significant differences among some groups (Fig. 4D). This result suggests that AUCPU is a more sensitive parameter. The AUCPU values were lower, but not significantly lower (p=0.34), in skin with 0.25% (w/w) Adr-mixed ointment application without PMS injections, than in skin with Adr-free TL ointment application. In the case of administration of the Adr-mixed TL ointment on the oedematous skin, although the 0.25% (w/w) Adr-mixed TL ointment showed no significant change in the AUCPU values (p=0.77), the 0.50% (w/w) Adr-mixed TL ointment produced a significant decrease in AUCPU values (p<0.01). The combined topical application of Adr with TL decreased the AUCblood values in skin irrespective of PMS injection status. As for the systemic absorption of TL, PMS injection significantly increased the AUCblood values (p>0.01) with the administration of the Adr-free TL ointment (Fig. 4E), even though the AUCPU values showed no change between those with or without the PMS injections. Furthermore, the 0.25% (w/w) Adr-mixed TL ointment did not decrease AUCblood values in the oedematous skin. The difference in profiles between skins with or without the PMS injection suggests that the increase in TL absorption from PMS injection cannot be explained by the skin blood flow only.
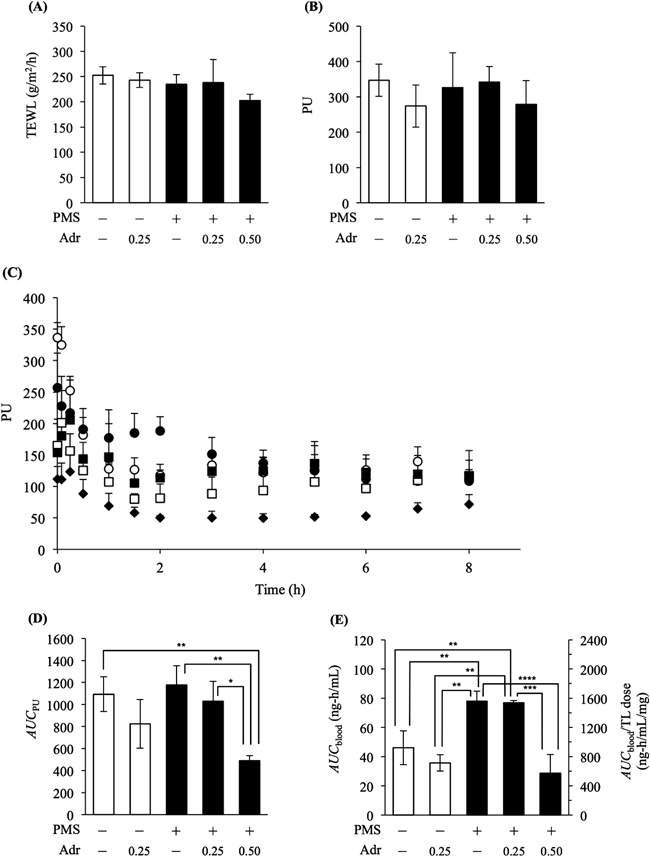
Symbols: open circle, PMS (−), Adr (−); open square, PMS (−), Adr (+, 0.25% (w/w)); closed circle, PMS (+), Adr (−); closed square, PMS (+), Adr (+, 0.25% (w/w)); closed diamond, PMS (+), Adr (+, 0.50% (w/w)). Each value is expressed as the mean±S.D. (n=3, 4); * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 between two groups, ANOVA followed by the Tukey–Kramer method.
In the case of co-application of 0.50% (w/w) Adr in the ointment, oedematous skin showed significantly lower AUCblood values when compared with the oedematous skin given Adr-free TL ointment. From these results, it may be inferred that the combined topical application of Adr with TL is useful in the prevention of systemic absorption of TL by diminishing skin blood flow.
Results from Figs. 4A, C and D were overlaid on Figs. 5A, B in order to separate and clarify the effect of administration of TL ointment mixed with Adr on systemic TL absorption from the relationships between TL absorption and SC barrier, and between TL absorption and skin blood flow. Figure 5A shows the relationship with TEWL. AUCblood data is presented as values divided by each TL dose, or each application area and concentration in ointment. The solid line is the regression curve in Fig. 1D (AUCblood/TL dose=(3.9702×TEWL)−34.893). Fully stripped skin and the skin with combined use of Adr (Adr, 0.25% (w/w)) have AUCblood values close to the calculated regression line. In contrast, the fully stripped skin with PMS injections exhibits AUCblood values above this line. Whilst combined use of Adr (Adr, 0.25% (w/w)) on oedematous skin shows AUCblood values above the line, increasing Adr concentration in the ointment to 0.50% (w/w) resulted in AUCblood values on or below the line. When considering the relationship with skin blood flow (Fig. 5B), in cases with fully stripped skin and PMS injection, the AUCblood values positively correlated to AUCPU (dashed line; r=0.835, p<0.05). AUCblood values in fully stripped skin with PMS injection are higher than that estimated from the correlation curve. Combined topical use of Adr (0.50% (w/w)) on the skin with PMS injection resulted in AUCblood values closely associated with or slightly below the correlation curve.

Symbols: open circle, PMS (−), Adr (−); open square, PMS (−), Adr (+, 0.25% (w/w)); closed circle, PMS (+), Adr (−); closed square, PMS (+), Adr (+, 0.25% (w/w)); closed diamond, PMS (+), Adr (+, 0.50% (w/w)). The solid line in (A) is the regression curve in Fig. 1D: (AUCblood/TL dose=(3.9702×TEWL)−34.893). The regression curve in (B) was calculated from data for open symbols; the r and p-value were calculated by the Pearson’s test.
Several studies have reported on the systemic absorption of TL following topical application of TL ointment on the skin of AD patients. In these studies, however, the skin tested was merely described as “lesional skin,” or was classified according to the extent or severity of AD.11,25–27) The skin of AD patients presents in different forms, including dryness, erythema, oedema, incrustation or haemorrhage. Thus, in order to understand permeation behaviour or systemic absorption of TL through altered skin, the skin structure or condition should be taken into consideration. In this study, we examined the relationship between systemic absorption of TL and alterations in physiological skin conditions that AD patients exhibit, such as disruption of SC, changes in skin blood flow and increases in vascular hyperpermeability.
Animal models have been developed and used widely in various fields of research regarding AD.28,29) Whilst there are several reports on the investigation of skin permeation and development of drug delivery systems in AD animal models,30–32) evaluation of pharmacokinetic properties is difficult due to the concurrent inflammatory reactions in AD skin. In order to investigate the drug disposition after topical application on AD skin, it is necessary to understand different influential factors and their individual effects. Thus, we prepared several skin models with different physiological conditions in hairless rats, in order to separate the complex inflammatory reactions into fundamental processes and evaluate the relationships between TL absorption and each process. The tape stripping treatment was performed to change SC barrier function, with a resultant increase in TEWL values. PMS was intradermally injected to induce an acute inflammatory reaction; oedema with plasma protein leakage was observed and attributed to vascular hyperpermeability. Although PMS injections did not alter TEWL and PU values, the inter-individual variation of TEWL and PU values (coefficient of variation (CV), 21.71 and 33.57%) were higher compared with that of the intact skin model (CV, 11.41 and 21.76%). These results suggest that the PMS injection did alter physiological skin conditions. In order to investigate the effect of skin blood flow, subcutaneous infusion of Adr was performed, with skin blood flow observed to decrease. These skin models were used for in vivo absorption studies.
No difference was observed in the systemic absorption of TL through intact skin or oedematous skin with intact SC (Table 1), suggesting that SC permeation of TL is the rate-limiting step in TL absorption following topical application, and that the disrupted SC barrier in AD patients increases TL absorption. The degree of disruption in the SC barrier was positively correlated with TL absorption (Fig. 1D). Previous studies have shown that TL absorption through intact skin has a positive linear relationship with the TL concentration of ointment, or the surface area of application in rats.33) In the present study, we found that TL absorption through fully stripped skin positively correlated with the TL concentration of ointment and the surface area of application (Figs. 1B, C). These findings suggest that systemic absorption of TL can be estimated by measuring TEWL, and with respect to mitigating systemic side effects, the dilution of the TL ointment according to patient TEWL value is an option in order to decrease the systemic absorption of TL. Considering the therapeutic efficacy of TL, the decrease in skin concentration of TL due to the dilution of ointment could be a matter of concern. In our preliminary experiment on an AD mice model, when TL ointments were diluted, the ratio of TL blood concentration to skin concentration was equivalent. The dilution of TL ointment as a means of avoiding excessive increases in systemic absorption of TL, does not mitigate therapeutic efficacy. The skin barrier function in AD patients is abnormal in both lesional areas and non-lesional areas, with increased TEWL.9) Thus, TL ointment should only be applied to lesional areas, and unnecessary application of TL ointment to non-lesional areas should be avoided, in order to minimize systemic absorption.
Following removal of SC by tape stripping, the intracutaneous injection of PMS increased the systemic absorption of TL (Fig. 2). Conversely, subcutaneous infusion of Adr decreased TL absorption, due to the reduced skin blood flow associated with vasoconstriction (Fig. 3C). Thus, the Adr-mixed TL ointment was used in an attempt to prevent TL absorption through fully stripped skin, with or without PMS injection. As shown in Figs. 4D, E, Adr-mixed ointment decreased skin blood flow and TL absorption in both skin models. Furthermore, the positive correlation between TL absorption and skin blood flow was observed in the fully stripped skin, without PMS injection (Fig. 5B). These results indicate that the systemic absorption of TL through the skin in absence of the SC barrier depends on skin blood flow. TL absorption in fully stripped skin with PMS injection exhibited higher values than that estimated by the correlation curve, suggesting that vascular hyperpermeability induced by the PMS injection also aided the systemic absorption of TL. Whilst vascular hyperpermeability from PMS injection could affect TEWL values, TEWL values show no change in Table 1 and Fig. 4A. The PU values also exhibited no significant change (Table 1, Fig. 4B). A similar rank order was observed between TEWL and PU values (Figs. 4A, B), suggesting that skin blood flow was the primary contributor to TEWL, with the effect of vascular hyperpermeability on TEWL not apparent. Interestingly, combined topical use of Adr (0.50% (w/w)) on the skin with PMS injection exhibited AUCblood values closely associated with or below the correlation curve (Fig. 5B). This suggests that the combined topical use of Adr prevents systemic absorption of TL by decreasing vascular hyperpermeability, as well as skin blood flow. Figure 5A shows the relationship between TEWL and the fully stripped skin with PMS injection, showing AUCblood values above the regression curve (solid line) in Fig. 1D. Although combined use of Adr (Adr, 0.25% (w/w)) on oedematous skin had no effect on AUCblood values above the line, an increase in Adr concentration in ointment to 0.50% (w/w) resulted in AUCblood values on or below the line. These results indicate that low blood flow limits TL absorption through the skin in acute inflammation with severe SC disruption.
In the case of intact SC, the SC barrier limited the systemic absorption of TL through the skin. SC disruption showed increasing TL absorption with increasing TEWL. With extreme disruption of the SC barrier, intradermal conditions, such as blood flow or vascular permeability, strongly affected TL absorption. In this case, the rate-limiting step in TL absorption is shifted from SC permeation to blood capillary uptake. However, the fully stripped SC in this study is an extreme condition, such as skin erosion in an equivalent, real world AD skin context. Essentially, TL ointment is used in cases where the skin continues to show SC barrier function, even to a very minor degree, and in these cases, SC permeation is a rate-limiting step for TL absorption. Therefore, estimation of TL absorption via measurement of TEWL is a reasonable methodology. Dilution of TL according to TEWL values may be useful from the viewpoint of mitigating systemic side effects. Finally, based on these results, we suggest an administration design for TL ointment that is tailored to various skin conditions, as seen in the flowchart (Fig. 6), in order to prevent systemic side effects. TL absorption depends on SC barrier function. When dysfunction of the SC barrier develops and a loss of barrier function occurs, attention should be directed to internal conditions, as TL absorption is aided by the acceleration of blood flow and/or vascular hyperpermeability. Therefore, assessment of SC barrier function by TEWL measurement is an important first step in judging the strategy of TL ointment therapy. The skin blood flow in chronic lesions exhibits no difference to non-eczematous skin.19) In this case, dilution of ointment may be an option to decrease TL absorption. In contrast, for AD patients with acute exacerbation of severely SC-disrupted skin, exhibiting redness or warmth, the combination use of vasoconstrictor could be beneficial in preventing excessive vascular uptake of TL. In these cases, simultaneous dilution of ointment according to patient TEWL values could also be effective.

In conclusion, the present study has shown that systemic absorption of TL following topical application of TL ointment is affected by physiological conditions of the skin. We used hairless rats with artificially altered physiological skin conditions. Comparison of TL absorption among the artificially altered skins revealed relationships between each alteration and the TL absorption property. The findings in this study support the recommendation that the assessment of the physiological conditions in lesional skin is important to provide an improved understanding of TL absorption in AD skin, and further examinations in AD animal models or patients are expected to help predict drug disposition and devise a treatment plan with topical formulations.
This work was supported by JSPS KAKENHI, Grant numbers 23590197 and 26460042.
The authors declare no conflict of interest.