2016 Volume 39 Issue 3 Pages 368-377
2016 Volume 39 Issue 3 Pages 368-377
Most safety evaluations of dry powder inhalers (DPIs) using cultured cells have been performed with dry powder formulations dissolved in a medium. However, this method is not considered to be suitable to evaluate the safety of inhaled dry powder formulations correctly since it cannot reflect the actual phenomenon on the respiratory epithelial surface. In this study, we established a novel in-vitro safety evaluation system suitable for DPIs by combining an air–liquid interface cultured cell layer and a device for dispersing dry powders, and evaluated the safety of candidate excipients of dry powders for inhalation. The safety of excipients (sugars, amino acids, cyclodextrins, and positive controls) in solutions was compared using submerged cell culture systems with a conventional 96-well plate and Transwell®. The sensitivity of the cells grown in Transwell® was lower than that of those grown in the 96-well plate. Dry powders were prepared by spray-drying and we evaluated their safety with a novel in-vitro safety evaluation system using an air–liquid interface cultured cell layer. Dry powders decreased the cell viability with doses more than solutions. On the other hand, dissolving the dry powders attenuated their cytotoxicity. This suggested that the novel in-vitro safety evaluation system would be suitable to evaluate the safety of DPIs with high sensitivity.
Pulmonary drug delivery has been investigated as not only a local treatment for respiratory diseases such as asthma or chronic obstructive pulmonary disease, but also as an alternative route for the systemic delivery of macromolecule drugs such as insulin, calcitonin, and heparin.1–4)
Among the three major systems for pulmonary delivery—nebulizers, pressurized metered-dose inhalers (pMDIs), and dry powder inhalers (DPIs)—DPIs have several advantages, including favorable portability, a low cost, and the lack of a need for propellants. Furthermore, the handling of DPIs is easier than that of pMDIs because of breath-actuated passive aerosolization.5)
For effective pulmonary deposition after inhalation, in general, the optimal aerodynamic diameter of drug particles is less than 6 µm.6,7) However, micronized drug particles tend to be highly cohesive and poorly flowable, leading to low-level performance. To solve these problems, it is usual for an excipient, such as coarse lactose monohydrate, to be formulated to physically attach to fine active ingredients.8,9) It has been recently reported that the addition of amino acids or cyclodextrins (CyDs) as excipients improves pulmonary delivery.10,11)
As for fundamental research on DPIs, evaluation of the physical properties such as particle size and pulmonary deposition is mainstream, whereas biological evaluation such as of the efficacy and safety is essential for clinical application.12) These biological evaluations of dry powder formulations are often conducted using in-vivo animal experiments or ex-vivo lung preparations.12,13) However, it is often the case that the experimental results become difficult to interpret accurately, because it is hard to precisely control the dose of dry powder formulations.14,15) In addition, it is not easy to evaluate the local biological effect of inhaled dry powder formulations on lung epithelial cell layers. Therefore, a need arises for an evaluation system using cultured cells that is simpler and easier to precisely evaluate the dose and efficacy of inhaled dry powder formulations.16) The conventional in-vitro biological evaluation of dry powder formulations using cultured cells has been performed using a submerged cell culture (SUB; Figs. 1a, b) system with the dry powder formulations dissolved in a medium.17,18) However, it is generally considered that DPIs exhibit the following behavior after inhalation: 1) dispersion, 2) delivery to pulmonary mucosa, 3) deposition, 4) dissolution, and 5) absorption. Therefore, the SUB system is not necessarily suitable for the accurate evaluation of inhaled dry powder formulations since it cannot reflect the actual condition on the lung epithelial surface, including the direct attachment of the powders followed by their dissolution. Thus, an air–liquid interfaced cell culture (ALI; Fig. 1c) system has attracted much attention as a novel tool for the drug permeation characterization of inhaled dry powder formulations in vitro.

ALI is also applicable to the safety evaluation of inhaled materials; however, evaluation is often performed with aerosols or gaseous compounds, and there are still few reports on dry powder formulations.19–24)
In this study, thus, to apply ALI to evaluation of the safety of dry powders, we compared the safety of several candidate excipients with two cell culture systems (ALI with Transwell® insert and SUB with a 96 well plate and Transwell® insert) and three safety evaluation methods 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, lactate dehydrogenase activity (LDH) assay, and propidium iodide (PI) dyeing. By the direct dispersion of powders onto the ALI system, we evaluated the safety of sugars, amino acids, and CyDs as candidate excipients that we consider would improve the pulmonary deposition of dry powders.
As candidate excipients, D-(+)-trehalose dehydrate (Tre), 2-hydroxypropyl-γ-cyclodextrin (HP-γ-CyD), L-phenylalanine (Phe), L-leucine (Leu), and L-isoleucine (Ile) were purchased from Sigma-Aldrich Co. (Deisenhofen, Germany) and lactose monohydrate (Lac), D-(−)-mannitol (Man), α-cyclodextrin (α-CyD), β-cyclodextrin (β-CyD), γ-cyclodextrin (γ-CyD), and 2-hydroxypropyl-β-cyclodextrin (HP-β-CyD) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). As positive controls for toxicity evaluation, sodium dodecyl sulfate (SDS) and doxorubicin hydrochloride (DOX) were purchased from Wako Pure Chemical Industries, Ltd. and hydrogen peroxide (H2O2) was purchased from Sigma-Aldrich Co. Fluorescein sodium salt (Fl-Na; Sigma-Aldrich Co.) was used as a reagent for the determination of powder deposition in vitro. For cell culture, Roswell Park Memorial Institute (RPMI) medium, fetal bovine serum (FBS), penicillin-streptomycin (final concentration: 100 U/mL penicillin and 100 µg/mL streptomycin), and trypsin–ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma-Aldrich Co. Clear 96-well Microtest™ Plate and Transwell® permeable filters (pore size: 0.4 µm, 1.12 cm2, Transwell® type) were purchased from Corning Inc. (Corning, U.S.A.).
Cell CultureHuman alveolar epithelial cancer (A549) cells were grown in 151.9 cm2 flasks with RPMI supplemented with 10% FBS and penicillin–streptomycin. The cells were incubated at 37°C in an atmosphere of 90% relative humidity and 5% CO2. The medium was changed twice a week in flasks and cells were passaged weekly using 3 mL of trypsin–EDTA.
On a 96-well plate, A549 cells were seeded at a density of 4×103 cells/well with 100 µL of medium. After 24-h incubation, the cells were ready for safety evaluation.
On Transwell®, A549 cells were seeded at a density of 2×105 cells/well with 500 µL of medium in the apical chamber and the basolateral chamber was filled with 1.5 mL of medium. After 2 d, the medium in the apical chamber was removed to interface the cell layer with air. The medium in the basolateral chamber was changed every 2 d. After 4–11 d, the cells were ready for safety evaluation.
The Apparatus for Dispersing the PowdersThe apparatus for dispersing the powders was assembled with a disposable sampling tube, a 1-mL syringe, a 3-way stopcock, and a disposable tip to load the powders (Fig. 2). The powders in the tip were dispersed by releasing air compressed in the syringe.25)

Dry powders were prepared using a spray dryer (SD-1000; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) with a two-fluid nozzle (inner diameter: 0.4 mm). A 1% (w/v) sample solution delivered at a rate of 5 mL/min was atomized at an air pressure of 150 kPa and the droplet was dried at an inlet air temperature of 120°C. SDS, a positive control, was mixed with Man at a ratio of 1 : 49 (SDS/Man). To determine the exposed dose of dry powders on the cell layer, dry powder containing 0.1% (w/w) Fl-Na was prepared.
Morphological Analysis of Dry PowdersEach dry powder was used to fill a disposable tip and dispersed onto the specimen mount by releasing compressed air (0.2 mL) in a syringe by opening a three-way stopcock connecting the disposable tip and syringe,26) and platinum-coated with a spatter at 30 mA, 90 s (JFC-1600, JEOL DATUM Solution Business Operations, Tokyo, Japan). A scanning electron microscope (SEM; JEOL Type JSM-T20, JEOL DATUM Solution Business Operations) was used to observe the morphology of dry powders.
Particle Sizing by Laser DiffractionThe particle size distribution was measured with a laser diffraction scattering method using a diffractometer with a dry dispersing unit (LMS-3000; Seishin Enterprise Co., Ltd., Tokyo, Japan). Then, the dry dispersing unit was operated at a constant air pressure of 0.4 MPa to disperse the dry powders into the laser beam. From the obtained accumulative particle size distribution, d10, d50, and d90 values were calculated.
Determining Exposed Dose of Dry PowdersDry powders containing 0.1% (w/w) Fl-Na were dispersed with the apparatus (compressed air volume: 0.2 mL) (Fig. 2) on an apical chamber of Transwell® without cells. The surface of the membrane was wetted with 150 µL of PBS to capture the powders, and the amount of powders trapped in the apical chamber was quantified using a spectrofluorometer (Ex: 490 nm, Em: 515 nm) to estimate the actually applied dose.
Safety EvaluationWith the 96-well plate, the medium was removed from the surface of the cells and one of the candidate excipients dissolved in the 200-µL medium containing FBS was added and incubated at 37°C for 48 h (SUB-conv.). With Transwell®, the medium in the basolateral chamber was replaced with a fresh medium and one of the candidate excipients dissolved in the 150 µL of medium without FBS was added (SUB-insert) or a dry powder was dispersed (ALI-insert) to incubate at 37°C for 48 h. SDS/Man, DOX, and H2O2 were used in validation of the safety evaluation method as a positive control. We calculated the lethal concentration (LC50) or lethal dose (LD50) as an index of the safety of excipients based on a nonlinear least-squares method for an equation (Eq. 1) derived from the Hill equation:
 | (1) |
At 48 h after the exposure of the sample with SUB-conv., the sample solution was removed from the cells and 100 µL of 0.5-mg/mL MTT solution was added to each well and incubated for 4 h. Subsequently, 100 µL of 10% SDS solution was added to each well and incubated at 37°C for 12–24 h in a shading state.
At 48 h after the exposure of the sample with SUB-insert or ALI-insert, the sample was removed from both the apical and basolateral chambers, 250 and 1000 µL of 0.5-mg/mL MTT solutions were added to the apical and basolateral chambers, respectively, and the well was incubated for 1 h. Subsequently, 250 µL of 10% SDS solution was added to each well and incubated at 37°C for 12–24 h in a shading state.
LDH AssayAt 48 h after the exposure of the sample with SUB-insert or ALI-insert, the sample solution in the apical chamber and culture medium in the basolateral chamber were recovered and we quantified lactate dehydrogenase activity with the Lactate Dehydrogenase Activity Colorimetric Assay Kit (BioVision, Milpitas, U.S.A.).
PI DyeingAt 48 h after the exposure of the sample with SUB-insert or ALI-insert, the cells were treated and suspended with trypsin–EDTA (300 µL/well). The suspension was centrifuged at 17000×g for 7 min, and the supernatant was discarded. The cells were resuspended with 50 µg/mL PI solution in PBS and the intensity of fluorescence derived from the PI intercalating in the DNA of dead cells was determined using a spectrofluorometer (Ex: 493 nm, Em: 630 nm).
Statistical AnalysisStatistical comparisons were made with a one-way ANOVA. Comparisons of means were performed with Dunnett’s test. The significance level was set at p<0.05.
Before starting the safety evaluation of candidate excipients, we preliminarily compared the three toxicity assay methods, MTT assay, LDH assay, and PI dyeing, with the positive controls.
On the MTT assay, all positive controls decreased the cell viability concentration-dependently, suggesting that the evaluation method was effective (Fig. 3).

Each value represents the mean±S.D. (n=3). ※※: p<0.01, compared with control.
The LDH assay was more sensitive than the MTT assay. However, no positive controls showed a concentration-dependent increase in LDH activity. Curiously, the increase in the concentration of SDS (0.001–0.1%) or DOX (0.08–2 mM) decreased LDH activity in the apical chamber. It is considered that the samples in the supernatant would interact with LDH as previously reported for SDS.27) On the other hand, LDH activity in the basolateral chamber was dependent on the applied concentration of SDS or DOX (Fig. 4). However, Tre and Man (50–800 mM) did not increase LDH activity in the basolateral chamber concentration-dependently.

LDH was assayed in apical solutions (a, c, e) or in basolateral solutions (b, d, f). Each value represents the mean±S.D. (n=3). ※: p<0.05, compared with control. ※※ p<0.01, compared with control.
On PI dyeing, the fluorescence intensity of 10% SDS and 200 µM H2O2 was not significantly different from that of the negative control (treated with only medium), suggesting that PI dyeing was not sensitive enough to perform the safety studies (Fig. 5).

Each value represents the mean±S.D. (n=3). n.s.: not significant, compared with control.
Comparing the results obtained using the three methods mentioned above, we concluded that the MTT assay was the best method for the evaluation of excipient toxicity with ALI and employed it throughout the present study.
Physical Characterization of Dry PowdersThe dry powders were prepared by the spray-drying method and their shapes were observed using SEM (Fig. 6). All sugar particles had a spherical shape, but CyD particles had a hollow on their surface. On the other hand, Phe and Leu particles had unique shapes, such as a ring or collapsed balloon. It has been reported that surface active materials such as cyclodextrins and hydrophobic amino acids reduced the surface activity of the droplets to make powders with dimples.28–30) Particle sizes (d50) of sugars and CyDs were approximately 3 µm, but those of amino acids and SDS/Man were larger (Table 1).

| (µm) | Lac | Tre | Man | Phe | Leu | Ile | α-CyD | β-CyD | HP-β-CyD | SDS |
|---|---|---|---|---|---|---|---|---|---|---|
| d10 | 1.77 | 1.70 | 1.89 | 2.49 | 2.30 | 2.68 | 1.90 | 1.37 | 1.95 | 2.67 |
| d50 | 2.77 | 2.55 | 2.96 | 4.22 | 7.71 | 5.05 | 3.09 | 3.01 | 2.98 | 5.60 |
| d90 | 4.66 | 4.09 | 4.88 | 7.04 | 17.80 | 9.27 | 5.18 | 5.93 | 4.70 | 10.90 |
In SUB-conv., the sugars decreased cell viability concentration-dependently and the safety of each sugar was at the same level when compared with the molar concentration base (Fig. 7a). This may indicate that the toxicity caused by Lac, Tre, and Man was based on the same mechanism, i.e., the increase in osmotic pressure; however, Scherliess reported that the toxicity of sugars were not explained by only osmotic pressure because higher molecular weight materials required higher molar concentrations to give the same toxicity.31) The maximum concentration of Lac was 200 mM because of the limited solubility in the medium used. On the other hand, the safety of Man was the lowest among sugars when compared with the mass concentration base (Table 2). The comparison of LC50 shown in Table 2 revealed that the sugars were the safest among the candidate excipients examined in the present study.

Each value represents the mean±S.D. (n=4). The exposure concentration led to a significant difference compared with the control (0 mM): (a) Lac: ≥100 mM (p<0.01), Tre: ≥200 mM (p<0.01), Man: ≥100 mM (p<0.01), (b) Phe: ≥1 mM (p<0.01), Leu: ≥20 mM (p<0.01), Ile: ≥20 mM (p<0.01), SDS: ≥0.00347 mM (p<0.01), and (c) α-CyD: ≥5.0 mM (p<0.05), ≥10 mM (p<0.01), β-CyD: ≥0.030 mM (p<0.05), ≥3.0 mM (p<0.01), γ-CyD: ≥40 mM (p<0.01), HP-β-CyD: ≥5.0 mM (p<0.05), ≥10 mM (p<0.01), HP-γ-CyD: ≥ 40 mM (p<0.01).
| LC50 | Lac | Tre | Man | Phe | Leu | Ile | α-CyD | β-CyD | γ-CyD | HP-β-CyD | HP-γ-CyD | SDS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUB-conv. | mM | >200 | 336 | 331 | 25.6 | >100 | >100 | 11.9 | 4.10 | 39.5 | 13.7 | 69.9 | 0.416 |
| mg/mL | >68.5 | 127 | 60.4 | 4.23 | >13.1 | >13.1 | 11.6 | 4.65 | 51.3 | 21.1 | 123 | 0.120 | |
| SUB-insert | mM | >200 | >800 | >800 | >150 | >150 | >150 | 62.3 | >15.0 | >120 | 83.4 | >120 | 1.070 |
| mg/mL | >68.5 | >303 | >146 | >24.8 | >19.7 | >19.7 | 60.6 | >17.0 | >156 | 129 | >211 | 0.309 | |
Amino acids decreased the cell viability concentration-dependently (Fig. 7b). The safety of Phe was the lowest among the amino acids, although the reason is still to be studied (Table 2).
CyDs markedly decreased the cell viability concentration-dependently (Fig. 7c), and the safety of CyDs was the lowest among the excipients examined with the molar concentration base (Table 2). γ-CyD is the safest among the natural CyDs examined (α-, β-, and, γ-CyD). Moreover, the CyDs with increased aqueous solubility (HP-β-CyD and HP-γ-CyD) were safer than the corresponding natural CyDs (β-CyD and γ-CyD, respectively). In general, CyDs cause structural changes in the cell membranes due to their solubilizing capacity for lipids; the capacity of γ-CyD is reportedly the lowest among the natural CyDs.32) The increased solubility of HP-γ-CyD might result in the decreased interaction with cell membrane components.
Safety Evaluation with SUB-InsertIn SUB-insert, sugars did not affect the cell viability even at exposure concentrations close to their saturated solubility (Fig. 8a).

Each value represents the mean±S.D. (n=3). The exposure concentration led to a significant difference compared with the control (0 mM): (a) Lac, Tre, Man: not significant, (b) Phe: 150 mM (p<0.01), Leu, Ile: not significant, SDS: ≥3.47 mM (p<0.01), and (c) α-CyD: ≥60 mM (p<0.01), HP-β-CyD: ≥80 mM (p<0.01), β-CyD, γ-CyD, HP-γ-CyD: not significant.
In amino acids, Phe caused a slight decrease in the cell viability at the exposure concentration close to its saturated solubility. On the other hand, the other amino acids did not affect the cell viability at the exposure concentration close to their saturated solubility (Fig. 8b).
In CyDs, α-CyD caused a decrease in the cell viability (Fig. 8c). HP-β-CyD also decreased the cell viability; however, it still appeared to be safer than β-CyD, although its limited solubility made it difficult to compare them at concentrations higher than 15 mM.
In all excipients, the sensitivity of SUB-insert was lower than that of SUB-conv. (Table 2). This result was also confirmed with human bronchial epithelial cancer (Calu-3) cells with Man (data not shown). However, Scherliess reported that cells grown in the Transwell® system were a little more sensitive than cells cultured in a 96-well plate with Lac.31) This might be due to the differences in the cultured condition and/or the assay method between Scherliess and our group. To determine the cause of the low sensitivity of SUB-insert, we examined the effect of the cell number, exposure time, presence of serum, and permeation of an excipient on cell viability.
Examination of Causes of Low Sensitivity of SUB-InsertBecause the initial cell density in the well of SUB-insert is about 15 times thicker than that of SUB-conv. and the cultivation period of SUB-insert is at least 4 times longer than that of SUB-conv., the cell conditions of these two systems may be different very much. To examine the effect of the cell number on the cell viability, we applied Man, Phe, and HP-β-CyD solutions onto the wells seeded with various numbers of cells (4, 8, and 20 ×103 cells/well) in the 96-well plate. The seeding cell number did not have a significant effect on the cell viability, suggesting that the contribution of the cell number to the low sensitivity of SUB-insert was small (Fig. 9).

Each value represents the mean±S.D. (n=4). The exposure concentration led to a significant difference compared with 4000 cells: (a) 8000 cells: 400 mM (p<0.05), 800 mM (p<0.01), 20000 cells: ≥200 mM (p<0.01), (b) 8000 cells: 20, 50 mM (p<0.05), 5, 10 mM (p<0.01), 20000 cells: 1 mM (p<0.05), 2, 5, 10, 50 mM (p<0.01), and (c) 8000 cells: 10 mM (p<0.05), 1, 5 mM (p<0.01), 20000 cells: ≤10 mM (p<0.01).
To examine the effect of the exposure time and presence of serum on cell viability, we varied cell exposure conditions (4 h with FBS, 4 h without FBS, and 48 h with FBS). As a result, the viability of cells exposed to Man solutions was basically not affected by the exposure time or FBS, although we observed their significant effects at a specific concentration (200 mM). Phe solutions showed no decrease in the cell viability at 4 h with or without FBS. However, the longer the exposure time, the significantly lower the cell viability. We speculate that the Phe should be uptaken by the cells to express its toxicity and the amount uptaken may increase with time. On the other hand, the viability of the cells exposed to HP-β-CyD solutions was affected not by the exposure time but by FBS (Fig. 10). HP-β-CyD has been reported to bind to albumin in plasma,33) which would attenuate the solubilizing capacity of HP-β-CyD in the present study. On the other hand, the absence of serum was not shown to increase the cell viability, suggesting that the contribution of serum to the low sensitivity of SUB-insert was small. As described above, the effects of serum and the exposure time on the cell viability were different among excipients.

Each value represents the mean±S.D. (n=4). The exposure concentration produced a significant difference compared with 4 h with FBS: (a) 4 h without FBS: 200 mM (p<0.05), 48 h with FBS: 200 mM (p<0.01), (b) 4 h without FBS: not significant, 48 h with FBS: ≥5 mM (p<0.01), and (c) 4 h without FBS, 48 h with FBS: ≥10 mM (p<0.01).
The excipients applied on the apical surface of SUB-conv. could penetrate and accumulate in the cells but could not permeate through them because the cells were seeded on the bottom of the wells. On the other hand, the excipients applied on the apical surface of SUB-insert could permeate through the cells. To examine the effect of the permeation of an excipient on the cell viability, we removed the medium from the basolateral chamber and added Man, Phe, and HP-β-CyD solutions to the apical chamber (SUB-insert without permeating). As a result, in both cases, the cell viability following the exposure to solutions in SUB-insert without permeating was lower than that in SUB-insert, suggesting that permeation of an excipient lowered the sensitivity of SUB-insert (Fig. 11). It is considered that the excipient exposed at a high concentration in the apical chamber permeated to the basolateral chamber and the excipient that accumulated in the cells was decreased. On the other hand, the cell viability following the exposure to Man solutions in SUB-insert without permeating was higher than that in SUB-conv., suggesting that being cultured in the insert affected the cell viability (Fig. 11). It is considered that the integrity of the cell layer and/or differentiation of cells cultured for 4–11 d in the insert decreased the sensitivity of cells.
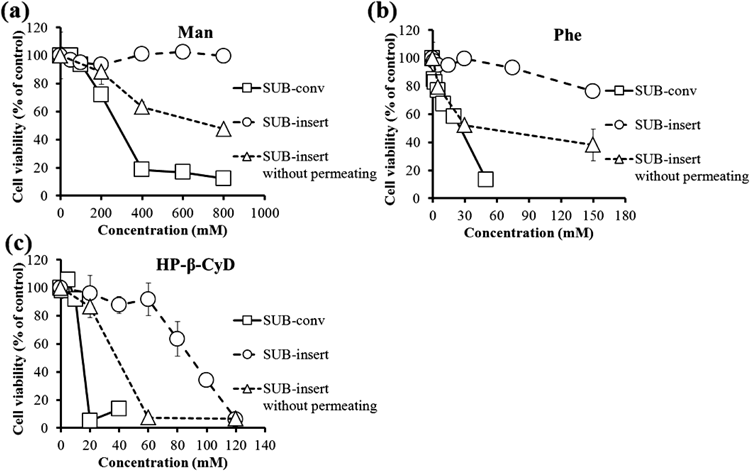
Each value represents the mean±S.D. (n=3). The exposure concentration led to a significant difference compared with SUB-insert without permeating: (a) SUB-conv.: ≥200 mM (p<0.01), SUB-insert: 800 mM (p<0.01), (b) SUB-insert: ≥5 mM (p<0.01), and (c) SUB-conv.: 20 mM (p<0.01), SUB-insert: 60 mM (p<0.01).
To evaluate the safety of the dry powders, we applied solutions and powders on cell layers on Transwell®. We confirmed visually that dry powders could be dispersed uniformly to apical chamber of Transwell® using the dry powder containing Fl-Na. In another experiment, the powders applied in the well were dissolved with 150 µL of medium without FBS. As a result, the Man powder decreased the cell viability compared with the solution (Fig. 12). On the other hand, the decrease in the cell viability due to the Man powder disappeared by dissolving it on the cell layer (Fig. 12). From these results, the decrease in the cell viability by the dry powder would not be due to a physical impact caused by the dispersed dry powder, but due to the dry powder itself remaining on the cell layer. It is considered that a high local Man concentration on the cell layer caused by the direct application of the powder would contribute to a decrease in the cell viability. The LD50 values for the cells in SUB-insert and ALI-insert applied with Man and the other candidate excipients were determined (Table 3). The decrease in the cell viability with the dry powders occurred with all the excipients except for Leu and Ile, the extent of which differed among the excipients (Table 3). Moreover, β-CyD powder did not decrease the cell viability so much. Leu, Ile, and β-CyD are low-level aqueous solubility excipients in the present study, which might affect the safety of the dry powders.

Each value represents the mean±S.D. (n=3). The exposure concentration led to a significant difference compared with solution: Powder, ≥2.67 mg/cm2 (p<0.01), Redissolution after addition: not significant.
| LD50 | Lac | Tre | Man | Phe | Leu | Ile | α-CyD | β-CyD | γ-CyD | HP-β-CyD | HP-γ-CyD | SDS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUB-insert | mg/cm2 | >9.19 | >40.5 | >19.5 | >3.32 | >2.64 | >2.64 | 8.11 | >2.28 | >20.8 | 17.2 | >28.3 | 0.143 |
| ALI-insert | mg/cm2 | 3.86 | 5.74 | 5.78 | 0.692 | >5.57 | >1.89 | 1.44 | >2.02 | 4.76 | 0.0132 | ||
Since the safety of the dry powders did not necessarily correlate with that of the solutions, it was suggested that ALI-insert may be more appropriate to evaluate the safety of dry powders. An in-vivo evaluation of the toxicity of the excipients with small animals will be useful to validate the appropriateness of the ALI-insert system.
To apply ALI to evaluate the safety of dry powders, in this study, we compared the results of safety evaluation among different evaluation systems with 96-well and Transwell® with the solutions of candidate excipients. The sensitivity of the cells grown in the Transwell® system (SUB-insert) was lower than that grown in the 96-well plate (SUB-conv.) which was partly attributed to the lower-level accumulation of excipients in SUB-insert. The safety of the dry powders evaluated with the cells grown in the Transwell® system (ALI-insert) was lower than that evaluated with SUB-insert, which was partly attributed to the higher concentration of the excipients dissolved in a small volume of mucus.
The degree of the decrease in safety of dry powders was shown to vary among excipients. This paper proposes that the safety of dry powders should be evaluated with an appropriate system such as ALI-insert.
The authors declare no conflict of interest.