2016 Volume 39 Issue 5 Pages 879-882
2016 Volume 39 Issue 5 Pages 879-882
Intraocular irrigating solution containing 1 µg/mL adrenaline is widely used during cataract surgery to maintain pupil dilation. Prepared intraocular irrigating solutions are recommended for use within 6 h. After the irrigating solution is admistered for dilution, the adrenaline may become oxidized, and this may result in a decrease in its biological activity. However, the stability of adrenaline in intraocular irrigating solution is not fully understood. The aim of this study was to evaluate the stability of adrenaline in clinically used irrigating solutions of varying pH. Six hours after mixing, the adrenaline percentages remaining were 90.6%±3.7 (pH 7.2), 91.1%±2.2 (pH 7.5), and 65.2%±2.8 (pH 8.0) of the initial concentration. One hour after mixing, the percentages remaining were 97.6%±2.0 (pH 7.2), 97.4%±2.7 (pH 7.5), and 95.6%±3.3 (pH 8.0). The degradation was especially remarkable and time dependent in the solution at pH 8.0. These results indicate that the concentration of adrenaline is decreased after preparation. Moreover, we investigated the influence of sodium bisulfite on adrenaline stability in irrigating solution. The percentage adrenaline remaining at 6 h after mixing in irrigating solution (pH 8.0) containing sodium bisulfite at 0.5 µg/mL (concentration in irrigating solution) or at 500 µg/mL (concentration in the undiluted adrenaline preparation) were 57.5 and 97.3%, respectively. Therefore, the low concentration of sodium bisulfite in the irrigating solution may be a cause of the adrenaline loss. In conclusion, intraocular irrigation solution with adrenaline should be prepared just prior to its use in surgery.
Phacoemulsification and aspiration, and extracapsular cataract extraction are performed more easily if mydriasis can be maintained until the intraocular lens has been inserted.1) If the pupil diameter becomes less than 7.0 mm, surgery becomes more difficult.2) It is important to maintain an optimal adrenaline concentration in the irrigating solution, especially for diabetic eyes where constriction of the pupil during ophthalmic surgery is more pronounced.3) Maintenance of mydriasis during the entire procedure is therefore crucial. This can be achieved with use of phenylephrine or tropicamide ophthalmic solutions before surgery. But most drugs are administered before surgery and flushed out of the eye by the intraocular irrigation solution.4) In this regard, intraocular irrigation with 1 µg/mL adrenaline provides a significant benefit in maintaining mydriasis during surgery. One advantage of using intraocular continuous irrigation with adrenaline, as opposed to a bolus, is that it continues to be exposed to the eye while the stimulus to miosis persists. Furthermore, there was no correlation between the flow rate of the irrigating solution and the rate of miosis when irrigation solutions containing adrenaline were used.5) A study also showed that intraocular irrigation with adrenaline kept the pupil well dilated and did not have an adverse effect on the patient’s pulse rate or blood pressure.6) Therefore, intraocular irrigation with 1 µg/mL adrenaline is widely used to maintain pupil dilation during surgery.6–8)
The irrigating solution kit consists of oxidized glutathione and its dilute solution at physiological pH and osmolality. Because the solution does not contain an antioxidant such as sodium bisulfite, the dissolved adrenaline is unstable. For clinical use, to prepare the ocular irrigation solution we added 0.5 mL of adrenaline injectable solution, including 500 µg/mL sodium bisulfite to 500 mL of irrigating solution. Therefore, the final sodium bisulfite concentration in the irrigating solution was 0.5 µg/mL. At such a low concentration, sodium bisulfite may not provide enough antioxidant activity for adrenaline.
Adrenaline is rapidly decomposed by oxidizing agents in aqueous solution. This process is increased by the presence of oxygen or transition metal ions, and by exposure to light, to alkaline pH, or to increases in temperature.9,10) The primary determinant of adrenaline stability is pH. Therefore, adrenaline stability in commercial preparations is maintained with sodium bisulfite and low pH (pH 2.3–5.0) to prevent oxidation. Edelhauser et al. reported that pH was an important factor affecting cell viability, and the optimal pH for corneal endothelial cells was reported to be pH 6.8–8.2.11) The pH of irrigating solution is 7.2–8.2. Although stable at low pH, increasing the pH after dilution by the irrigating solution will cause oxidation of adrenaline that may result in a decrease in its biological activity. Thus, we prepared the ocular irrigating solution with adrenaline just prior to its use in surgery. However, due to the surgical procedures, it is often not used immediately after reconstitution. If ophthalmologists fail to maintain patient mydriasis during surgery from an adrenaline concentration decrease with time, the surgeon’s impaired visualization through the small pupil may increase the risk of damage to the iris, may cause incomplete clearance of soft lens matter, or more importantly, could result in the rupture of the posterior capsule.6) The influences of pH and other antioxidants on adrenaline stability have not been systematically explored. In this study, we examined the stability of adrenaline in irrigating solution to provide information on optimal storage conditions.
The following drug products were used: balanced salt solution enriched with bicarbonate, dextrose, and glutathione (BSS PLUS™ 500 intraocular irrigating solution kit; Alcon, Tokyo, Japan), adrenaline injectable solution [BOSMIN™ injection (1 mg); Daiichi Sankyo, Tokyo, Japan], (R)-(−)-adrenaline (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and sodium bisulfite (Wako Pure Chemical Industries, Ltd.). All other materials used were commercially available and were analytical grade reagents.
Quantitative Determination of Adrenaline in the Irrigating Solution12)The HPLC system (LC-10 AT; Shimadzu, Kyoto, Japan) was connected with an electrochemical detector (ECD-100; EICOM, Kyoto, Japan) using a C18 reverse-phase column (5 µm, 150×3.0 mm, EICOM). The mobile phase consisted of citrate–acetate buffer, 4% octane–sulfonic acid, and 0.05% disodium ethylenediaminetetraacetic acid (EDTA), in 15% methanol, pH 2.8, and was pumped at a rate of 0.5 mL/min. The detector potential was set at 750 mV. Standard samples containing 100, 250, 500, and 1000 ng/mL of adrenaline diluted in 0.1 M hydrochloric acid were used to determine the linearity of drug recovery. The resulting standard curve was linear over the range of concentrations tested (r2=0.999 for adrenaline). The calibration curve of the peak area versus concentration was found to be linear and was reproducible over the evaluated range from 100 to 1000 ng/mL.
Preparation of Irrigating Solution with Adrenaline and Sodium BisulfiteInjectable adrenaline solution was added to the reconstituted irrigating solution just prior to the experiment. One-half milliliters of adrenaline at 1 mg/mL was diluted into 500 mL of irrigation solution to prepare an adrenaline concentration of 1 µg/mL. The adrenaline injectable solutions were within a range of pH 2.3–5.0 to prevent oxidation of adrenaline. The irrigating solution kit pH range was 7.8–8.2, according to the product information provided by the manufacturer. The pH of the solution was determined using a pH meter (Digital pH Meter V Series HM-30V; DKK-TOA, Tokyo, Japan). The samples were stored at room temperature (15–25°C), under a fluorescent light, for 24 h.
Sodium bisulfite (1 mg/mL) was added as a stabilizer to prevent oxidation of adrenaline at 0.5, 5, 50, and 500 µg/mL. The adrenaline concentration of each solution was measured during the 24 h time period.
Data AnalysesAll experimental measurements were collected in triplicate immediately after mixing. Each sample was assayed immediately to avoid further degradation. The stability of adrenaline is shown as a percentage of the starting concentration. Values are expressed as the mean±standard deviation (S.D.). Stability was defined as no detectable change in solution color or clarity. The pH of each sample was determined using a pH meter. A paired Student’s t-test was used to evaluate statistical differences between means when only two groups were evaluated. A p value <0.01 was considered statistically significant.
The pH of the irrigating solution just after adding adrenaline was pH 7.4. Changes in the pH after dilution by the irrigating solution may cause oxidation of adrenaline, and result in a decrease of its biological activity. Therefore, we evaluated the stability of the adrenaline in the irrigating solution at different pH. Each adrenaline solution was initially adjusted to pH 7.2, 7.5, or 8.0 by the addition of sodium hydroxide or hydrochloric acid. Sampling points were 1, 2, 3, 4, 5, 6, 9, 12, and 24 h after the preparation of the mixtures. With all pH conditions, no significant change of the starting pH was observed until 24 h, although the clear and colorless adrenaline solution turned pink after 24 h only at pH 8.0 (Supplementary Table 1).
Figure 1 shows the changes in adrenaline concentration at each pH. The adrenaline concentration (expressed as the percentage remaining) decreased depending on pH and time. The percentage adrenaline remaining 1 h after admixture to the irrigating solution was 97.6%±2.0 (pH 7.2), 97.4%±2.7 (pH 7.5), and 95.6%±3.3 (pH 8.0) of the initial concentration. More than 90% of the adrenaline remained after 6 h of mixing the irrigating solution with adrenaline at pH 7.2 and 7.5. However, at pH 8.0, the percentage decreased to approximately 65% of the initial concentration. Furthermore, after 24 h the percentages of remaining adrenaline were 66.8±2.5% (pH 7.2), 64.1±3.5% (pH 7.5), and 35.4±2.7% (pH 8.0) of the initial concentration. In the pH 8.0 solution, in addition to a remarkable percentage decrease of adrenaline, the color of the solution changed from colorless to pink. The appearances of the adrenaline solutions at pH 7.2 and 7.5 did not change during 24 h (Supplementary Table 1).
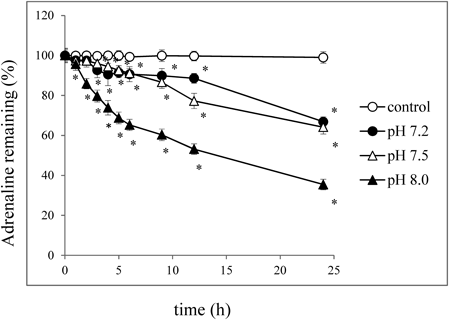
Irrigating solutions at pH 7.2 (—●—), pH 7.5 (—△—), and pH 8.0 (—▲—) containing adrenaline (1 µg/mL), or adrenaline injectable solution (1 mg/mL, pH 2.3–5.0) as a control were incubated at room temperature for 24 h. Each value was expressed as the mean±S.D. of three replicates: * p<0.01 compared with initial amount.
The manufacturer’s product information recommends using the irrigating solution kit within 6 h after mixing the glutathione with the irrigating solution. Therefore, the irrigating solution with adrenaline is commonly used within 6 h despite the lack of data regarding the adrenaline stability. To investigate the influence of sodium bisulfite on adrenaline stability in irrigating solution, the irrigation solutions (pH 8.0) with 1 µg/mL adrenaline containing 0.5 to 500 µg/mL of sodium bisulfite were prepared and the percent remaining adrenaline was measured. The adrenaline percentages remaining in pH 8.0 irrigating solution containing 0.5, 5, 50, or 500 µg/mL sodium bisulfite were 57.5, 57.9, 61.2, and 97.3%, respectively, at 6 h after mixing, which was the given expiration time of the irrigating solution kit (Fig. 2). Quantitative determination of the amount of adrenaline in each sample was obtained from the calibration curve. The peak area ratio of adrenaline compared with an internal standard substance was calculated and the percentage adrenaline remaining just after adding the mixture to the irrigating solution with 0.5 µg/mL of sodium bisulfite was 83%.
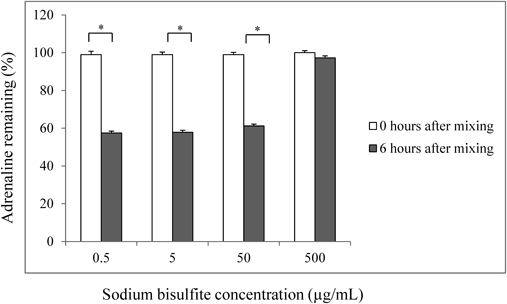
Adrenaline (1 µg/mL) in irrigating solution at pH 8.0 containing various concentrations of sodium bisulfite: 0.5, 5, 50, and 500 µg/mL were incubated at room temperature for 6 h. * p<0.01.
We have shown that the amount of adrenaline immediately decreased to 83% after adding the irrigating solution. According to the manufacturer’s description, the dilution of adrenaline to 1 : 100 with saline (0.9% NaCl without sodium metabisulfate) solution results in degradation to 62.3% of the original amount immediately after diluting. The degradation of adrenaline with irrigating solution is less than with saline. This result suggests that oxidized glutathione has an anti-degradation activity for adrenaline preservation. However, further studies are needed to confirm this finding to characterize its molecular mechanism. The dilution of the sodium bisulfite, added as stabilizer, and factors other than the rise of pH, can be considered as additional causes of the adrenaline concentration decrease. The sodium bisulfite added as the preservative in the adrenaline injection solution does not damage the corneal endothelium because of its low concentrations,6) therefore, intraocular irrigation with the standard 1 µg/mL adrenaline solution is widely used in the clinic. To investigate the influence of dilution of sodium bisulfite, various concentrations were added to the adrenaline irrigating solution (1 µg/mL), and changes in adrenaline concentrations were investigated. Decreases in the remaining adrenaline were not seen with 500 µg/mL sodium bisulfite, which is the same concentration as in the adrenaline preparation. At 0.5 µg/mL sodium bisulfite (the concentration currently used clinically), the percentage remaining adrenaline decreased notably. Therefore, dilution of sodium bisulfite is a cause of the adrenaline loss in the irrigating solution.
The irrigating solution kit is supplied in two packages for reconstitution prior to use, because of the instability of oxidized glutathione. According to the manufacturer’s instructions, the reconstituted irrigating solution itself is to be used within 6 h of mixing. As shown in the present study, the adrenaline concentration decreases depending on time after mixing. The percentage adrenaline remaining decreased to 90% of the control, or less, even at 2 h after the preparation (Fig. 1). Our results strongly suggest that intraocular irrigation solution containing adrenaline should be prepared just prior to use in surgery. If not used immediately after reconstitution, due to surgical procedures, the adrenaline biological activity could decrease, and therefore, additional adrenaline may be required.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.