2019 Volume 42 Issue 1 Pages 57-65
2019 Volume 42 Issue 1 Pages 57-65
CXC chemokine ligand 10 (CXCL10) is a CXC chemokine family protein that transmits signals by binding to its specific receptor, CXCR3. CXCL10 is also known as an interferon-γ-inducible chemokine involved in various biological phenomena, including chemotaxis of natural killer (NK) cells and cytotoxic T lymphocytes, that suppress tumor growth and inhibition of angiogenesis. In this study, we examined the effects of forced expression of CXCL10 in a murine colon carcinoma cell line (CT26) on growth and metastasis in syngeneic mice. We first established CT26 cells that were stably expressing murine CXCL10 (CT26/CXCL10) and compared their growth with their parental CT26 cells in vitro and in vivo. The in vitro growth of the CT26/CXCL10 and CT26 cells was comparable, whereas the in vivo growth of the CT26/CXCL10 cells in the skin was strongly suppressed. Liver metastasis of the CT26/CXCL10 cells was also significantly suppressed after intra-splenic implantation. Removal of NK cells by the administration of anti-asialo GM1 antibody canceled the suppression of subcutaneous growth and liver metastasis of CT26/CXCL10 cells. Immunofluorescence clearly showed that abundant NKp46-positive NK cells were recruited into the liver metastatic lesions of the CT26/CXCL10 cells, consistent with specific NK cell migration towards the culture supernatant from the CT26/CXCL10 cells in the chemotaxis assay using transwells. These findings indicate that CXCL10 prevents in vivo growth and metastasis of colon carcinoma cells by recruiting NK cells, suggesting that forced expression of CXCL10 in the colon tumors by gene delivery should lead to a favorable clinical outcome.
Chemokines are low-molecular-weight soluble proteins that interact with their cognate G-protein-coupled receptors that play important roles in immune cell trafficking and lymphoid tissue development.1,2) A chemokine receptor interacts with various chemokines, and similarly, a chemokine interacts with multiple chemokine receptors.3) Chemokines are classified into four groups, CC-, CXC-, C- and CX3C-, based on the conserved cysteine residues near their N-terminus.3)
In the tumor microenvironment, chemokines play multiple roles that lead to favorable and unfavorable clinical outcomes, such as immune cell recognition of tumors and tumor metastasis, respectively.4) Among various chemokines, CXC chemokine ligand (CXCL) 9, CXCL10 and CXCL11 bind to a common chemokine receptor (CXCR3) that is specifically expressed on CD8+ cytotoxic T lymphocytes (CTLs), Th1 cells and natural killer (NK) cells that attack tumor cells in the tumor microenvironment. Therefore, these chemokines are regarded as efficient effector molecules for anti-tumor immunity.5,6)
CXCL10 is an interferon (IFN)-γ-inducible chemokine that plays an important role in anti-tumor immunity together with interleukin (IL)-12 that is produced from dendritic cells and macrophages.7,8) NK cells are one of the most important effector cells in the initial protective response against tumors. NK cells also attack dormant tumor cells that evade recognition and anti-tumor immunity by CTLs.9) In this regard, prior studies have investigated CXCL10 for its potential use in tumor immune therapy. In several experimental models, CXCL10 reportedly had anti-tumor activities. For example, overexpression of CXCL10 in lymphomas induced their necrosis and, in melanomas, blocked their proliferation.10,11)
However, care should be taken when using chemokines in tumor immune therapy because they not only induce immune cell trafficking but also tissue-specific metastasis of certain tumor cells.12) For example, the interaction between CXCL12 and its receptor CXCR4 mediates metastasis and malignant transformation of certain tumor cells; e.g., breast, colorectal, and osteosarcoma cancer cells.13–15) CXCR3 reportedly contributed to the metastasis of certain colon carcinoma cells to the lung.16) Therefore, it is important to clarify the tissue- and cell type-specific action of chemokines, as well as their roles in tumor immunity and tumor metastasis before their clinical use.
In the present study, we examined the effects of forced expression of CXCL10 in colon carcinoma cells on their in vivo proliferation and metastasis. Although significant clinical progress has been made in treating primary tumors in the colon, metastasis has been regarded as a major risk factor of colon carcinoma patients. Patients with colon carcinoma often develop initial metastases in the liver, because cancer cells leaving a primary colon tumor enter the hepatic-portal system and are taken first to the sinusoids of the liver.17) In this study, we investigated the effects of CXCL10 on liver metastasis and its mechanism of action using murine colon carcinoma cells.
The CT26 (CT26.WT) colon carcinoma cells purchased from American Type Culture Collection (ATC C) were routinely maintained in RPMI-1640 (Sigma-Aldrich, U.S.A.) containing 10% (v/v) fetal bovine serum (FBS), 10 mmol/L N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), 100 units/mL penicillin and 100 µg/mL streptomycin. The cDNA of murine CXCL10 was amplified with the following primers: forward, 5′-CAT CAG CAC CAT GAA CCC AAG T-3′ and reverse, 5′-CGT GGC TTC ACT CCA GTT AAG G-3′. The CXCL10 cDNA was cloned into mammalian expression vector pcDNA3.1. pcDNA3.1-CXCL10 was transfected into CT26 cells by electroporation using the Neon transfection system (Thermo Fisher Scientific, U.S.A.), and the cells that were stably expressing CXCL10 (CT26/CXCL10 cells) were selected by G418 (1000 µg/mL, Nacalai Tesque, Japan). The expression of CXCL10 from the expression vector in CT26/CXCL10 cells was confirmed by RT-PCR using a forward primer corresponding to the sequence of the T7 promoter (5′-TAA TAC GAC TCA CTA TAG GG-3′), and the reverse primer that was used for CXCL10 cDNA amplification described above. NK-92 cells purchased from ATC C were routinely maintained in RPMI-1640 containing 10% (v/v) FBS, 10 mmol/L HEPES, 100 units/mL penicillin, 100 µg/mL streptomycin, non-essential amino acids, 100 µmol/L 2-mercapto ethanol and 10 ng/mL human IL-2.
Seven- to eight-week-old female BALB/c mice (Charles River, Japan) were used for in vivo experiments. All animal studies were approved by the Chiba University Animal Care Committee.
RT-PCRTotal RNA was extracted from each cell line using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. RT-PCR was performed with PrimeScript RT reagent kit with gDNA Eraser (TaKaRa Bio, Japan).
Western Blot AnalysisCT26 or CT26/CXCL10 cells cultured to confluent in 60-mm diameter dishes and their culture supernatants were collected. Recombinant murine CXCL10 (Peprotech) was used as a positive control. Proteins were resolved by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Hybond-P, GE Healthcare, U.S.A.). Membranes were blocked with 5% skim milk in 50 mM Tris–HCl, 150 mM NaCl, pH 7.5 containing 0.05% Tween 20 overnight at 4°C and incubated with biotinylated anti-CXCL10 antibody (1 µg/mL, rabbit antibody, catalogue number 500-P129BT, Peprotech, U.S.A.) for 1 h at room temperature. After incubation with horseradish peroxidase-streptavidin (diluted 1 : 2000, catalogue number 405210, BioLegend, U.S.A.) for 1 h, CXCL10 on the membranes was detected by using ECL Advance (GE Healthcare) and ImageQuant LAS 4000 (GE Healthcare).
Tumorigenicity AssayMice were given subcutaneous injections of 3 × 105 CT26 or CT26/CXCL10 cells into the right flanks in a total volume of 0.1 mL. Tumor growth was evaluated every other day by measuring the two perpendicular diameters with a digital caliper. Subcutaneous tumor volumes were calculated in mm3 as 0.5 × (width)2 × (length). Subcutaneous tumors were dissected on day 21 and tumor volumes were calculated in mm3 as (width) × (length) × (height) × π/6 based on the ellipsoid formula. Metastatic liver tumors were established by injecting 1 × 105 CT26 cells in 50 µL phosphate buffered saline (PBS) into spleens.18) Three weeks later, livers were taken out, and visible liver nodules were counted. The tissues were embedded in Optimal Cutting Temperature (OCT) compound (Sakura, Japan) and stored at −80°C until use in immunofluorescence.
In order to deplete NK cells in mice, anti-asialo GM1 antibody (10 mg/mL, catalogue number 986-10001, Wako Pure Chemical Industries, Ltd., Osaka, Japan) was diluted 1 : 20 and intraperitoneally injected into mice. Starting on the day before tumor implantation into the right flanks or spleens, 200 µL per mouse was injected once every 5 d. In these experiments, mice inoculated with tumors into their spleens were sacrificed on day 18 for humane reasons.
In Vitro Growth AssayCT26 and CT26/CXCL10 cells were trypsinized, washed and plated in triplicate in 96-well plates at five thousand cells per well in the culture medium. After a 24-h incubation at 37°C, cell viability was determined using Cell Counting Kit-8 (Dojindo, Japan). The absorbance at 450 nm was measured using a microplate reader Model 680 (Bio-Rad, U.S.A.).
ImmunofluorescenceSerial frozen sections of 7 µm in thickness were analyzed by immunofluorescence. Sections were fixed with ice-cold acetone, blocked with 3% BSA in PBS (Nakalai Tesque) and incubated with anti-NKp46 antibody (10 µg/mL, goat antibody, catalog number AF2225; R&D Systems, U.S.A.) or control goat immunoglobulin G (IgG) (10 µg/mL, Sigma-Aldrich) used as an isotype control and 4′-6-diamidino-2-phenylindole (DAPI) (0.5 µg/mL, Roche Applied Science, U.S.A.). Subsequently, the sections were incubated with donkey anti-goat IgG coupled to Alexa Fluor 594 (2 µg/mL, Thermo Fisher Scientific). Cryosections were analyzed by a fluorescence microscopy BZ-9000 (KEYENCE, Japan).
Hematoxylin and Eosin StainingThe left lateral lobe of each liver was cut and embedded in OCT compound. Frozen blocks were cut into 7-µm sections. Each section was fixed with 4% formaldehyde (Wako Pure Chemical Industries, Ltd.) and stained with hematoxylin and eosin (Wako Pure Chemical Industries, Ltd.). The percentage of the area occupied by tumor in each section was determined using ImageJ software.19)
Chemotaxis AssayNK-92 cells (1 × 105 cells/well) were added to the polycarbonate filters (Chemotaxicell, 8.0 µm, Kurabo, Japan) that had been set on the wells of 24-well cell culture plates containing various concentrations of recombinant murine CXCL10 (mCXCL10, Peprotech) or the supernatant of CT26 or CT26/CXCL10 cells. After incubation at 37°C for 3 h, the cells that had not passed through the filters were wiped out with cotton swabs. Cells that had migrated to the lower surface of the filters were fixed with 4% formaldehyde and stained with 0.1% crystal violet (Wako Pure Chemical Industries, Ltd.). The number of stained cells was counted in five random fields of pictures taken by BZ-9000 (KEYENCE). In some experiments, NK-92 cells were incubated with 5 µg/mL anti-CXCR3 antibody (catalogue number 353702, BioLegend) for 30 min before and during the chemotaxis assay.
Statistical AnalysisData were presented as the mean ± standard deviation (S.D.). Statistical analysis was performed with Student’s t-tests. Differences were considered to be statistically significant at p < 0.05.
Liver metastasis is one of the worst risk factors of colon carcinoma. We used murine colon carcinoma CT26 cells20,21) to examine the effects of CXCL10 expression on tumor growth and liver metastasis. We first amplified cDNA encoding murine CXCL10 and cloned it into a mammalian expression vector pcDNA3.1. The expression vector was introduced into CT26 cells by electroporation, and the cells were selected by G418. The resultant CT26 cells overexpressing murine CXCL10 (Fig. 1A) were termed CT26/CXCL10 cells. Western blot analysis (Fig. 1B) indicated that CT26/CXCL10 cells secrete approximately 3 times more CXCL10 than CT26 cells (0.796 ng/mL in CT26 supernatant vs. 2.43 ng/mL in CT26/CXCL10 supernatant).

CT26 cells (3 × 105 cells in 100 µL PBS per mouse) were subcutaneously inoculated into BALB/c mice (n = 5). (A) The expression of CXCL10 mRNA in CT26/CXCL10 cells was determined by RT-PCR. pcDNA3.1-CXCL10 expression vector was used as a positive control (Plasmid). (B) CXCL10 released into culture supernatant was detected by Western blot analysis. Concentration of CXCL10 in each supernatant is indicated. (C) Tumor size of right flank was monitored every other day. Dissected tumor volume (D) and weight (E) on day 21. (F) Cell viability determined by the Cell Counting Kit-8. (G) Pictures were taken on day 21 to compare the size of the tumors (indicated by the arrow heads). Data are expressed as means ± S.D. p values were determined by two-tailed Student’s t-test. ns, not significant. * p < 0.05. (Color figure can be accessed in the online version.)
To assess the effects of CXCL10 expression in the murine colon carcinoma cells on their growth in vivo, we inoculated parental CT26 cells and CT26/CXCL10 cells into the right flanks of BALB/c mice. The tumor size was measured using a digital caliper, and the volume was determined with the following equation: 0.5 × (the longest diameter) × (orthogonal width)2 (Fig. 1C). Three weeks after the inoculation of the cells, the tumors were taken out from the mice and their volumes (L × W × H × π/6, Fig. 1D) and weights (Fig. 1E) were determined. Although the in vitro growth of CT26 and CT26/CXCL10 cells was comparable (Fig. 1F), large tumors were observed in mice inoculated with CT26 cells, whereas no tumors were observed in 3 out of 5 mice inoculated with CT26/CXCL10 cells, and only small tumors were observed in the remaining 2 mice (Fig. 1G). Consistent with these findings, the tumor volume (Fig. 1D) and weight (Fig. 1E) of CT26/CXCL10-inoculated animals were significantly less than those of CT26-inoculated animals. These results indicate that CXCL10 overexpression inhibits the in vivo growth of CT26 cells but not the in vitro growth of those cells.
NK Cells Are Required to Prevent Subcutaneous Tumor Formation of CT26/CXCL10 CellsTo determine whether NK cells are required for the suppression of subcutaneous tumor formation of CT26/CXCL10 cells described above, we next pre-administered anti-asialo GM1 antibody which depletes NK cells in mice22) before the tumor cell inoculation. When CT26 and CT26/CXCL10 cells were inoculated in the flanks after anti-asialo-GM1 antibody treatment, both of the cells formed comparable size of tumors with no statistical difference (Fig. 2), suggesting that NK cells play an important role in the rejection of CT26/CXCL10 cells observed in Fig. 1.
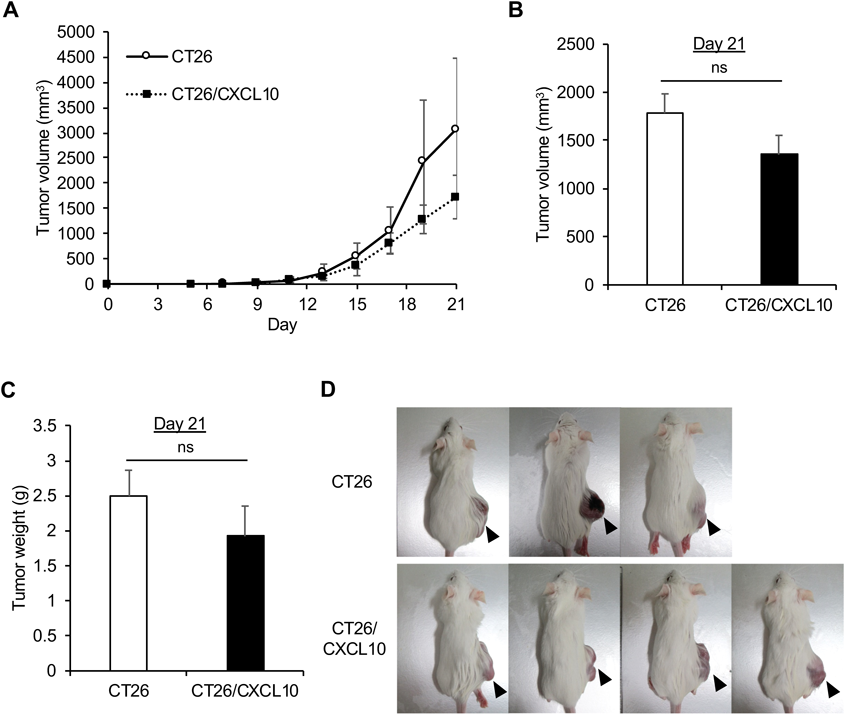
In order to deplete NK cells, anti-asialo GM1 antibody was intraperitoneally injected into mice starting from the day before tumor implantation and, thereafter, once every 5 d. CT26 and CT26/CXCL10 cells (3 × 105 cells in 100 µL PBS per mouse) were subcutaneously inoculated into BALB/c mice (n = 3 for CT26, n = 4 for CT26/CXCL10). (A) Tumor size of right flank was monitored every other day. Dissected tumor volume (B) and weight (C) on day 21. (D) Pictures were taken on day 21 to compare the size of the tumors (indicated by the arrow heads). Data are expressed as means ± S.D. p values were determined by two-tailed Student’s t-test. ns, not significant. (Color figure can be accessed in the online version.)
The CT26 cells are known to metastasize through the portal vein to the liver.23) We next examined the effects of forced expression of CXCL10 in CT26 cells on their liver metastasis. The CT26 and CT26/CXCL10 cells were implanted into the spleens of BALB/c mice, and the liver metastasis was determined 3 weeks after implantation (Fig. 3A). The number of tumor nodules on the liver surface of the CT26/CXCL10-transplanted mice was considerably smaller than that of the CT26-transplanted mice (Figs. 3B, C, p = 0.075). In addition, there was a tendency for the liver weight of the CT26/CXCL10-transplanted mice to be smaller than that of the CT26-transplanted mice (Fig. 3D, p = 0.059).
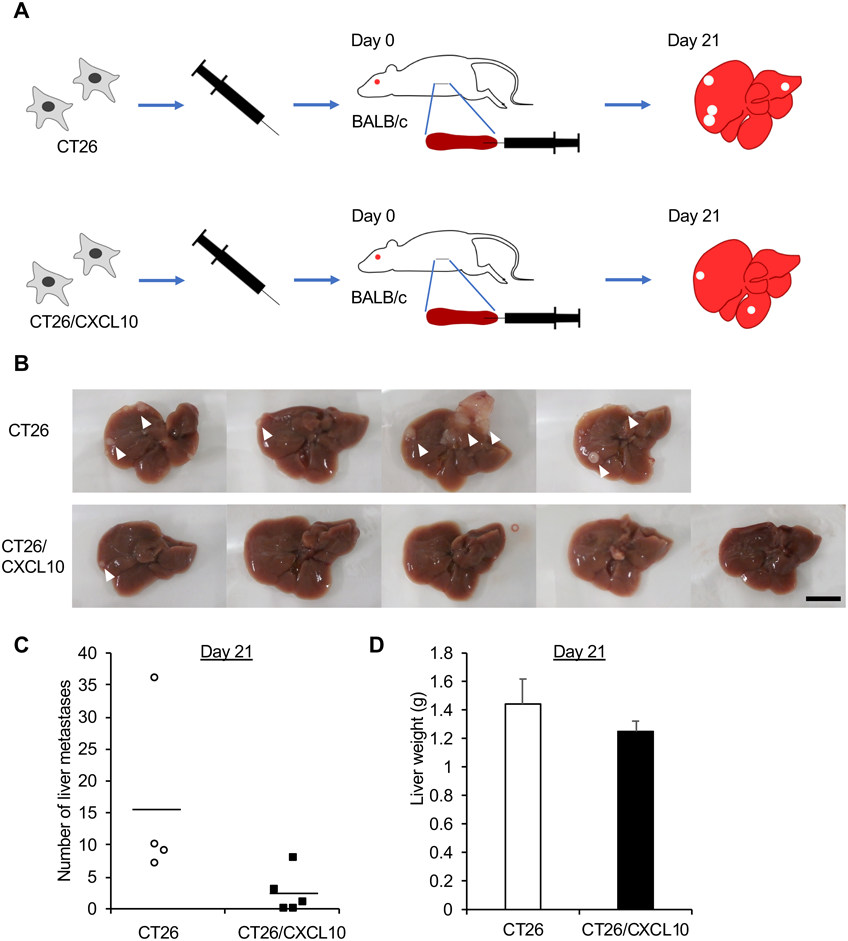
(A) CT26 cells or CT26/CXCL10 cells (1 × 105 cells in 50 µL PBS per mouse) were inoculated into spleens of BALB/c mice (n = 4 for CT26, n = 5 for CT26/CXCL10) on day 0. (B) Livers were harvested 21 d after tumor inoculation into the spleens and the pictures were taken to compare the size of the tumors (indicated by the white arrow heads). Scale bar, 1 cm. (C) The number of tumor nodules in the livers were counted on day 21. (D) The liver weight on day 21. Data are expressed as means ± S.D. (Color figure can be accessed in the online version.)
We subsequently examined the tumors inside the liver by staining the frozen sections with hematoxylin and eosin. The left lateral lobe, where metastasis was primarily observed, was cut into four pieces, and the cut surface was placed toward the bottom of the cryomold and embedded in the OCT compound to make frozen blocks (Fig. 4A). Three frozen sections were made with 200-µm intervals and stained with hematoxylin and eosin (Fig. 4B). As shown in Fig. 4B, tumors in the liver tissues of mice transplanted with CT26 cells were clearly seen (highlighted with white dashed line), whereas no tumors were seen in mice transplanted with CT26/CXCL10, although microtumors were occasionally seen in the latter. The percentage of tumor areas in each piece of the liver section was determined with ImageJ software and plotted in Fig. 4C. This semi-quantitative analysis indicates that the liver metastasis of CT26/CXCL10 was significantly lower than that of CT26 parental cells (p = 0.022). These data indicate that the expression of CXCL10 suppressed liver metastasis of CT26 cells.

(A) The left lateral lobe of each liver was cut into four pieces and embedded in OCT compound. Frozen blocks were cut into 7-µm sections. (B) Sections were stained with hematoxylin and eosin. Scale bars, 300 µm. Tumor is surrounded by the white dashed line. (C) The percentage of the area occupied by tumor in each section was determined using ImageJ software. Each dot represents the percentage of tumors in each piece of the liver section and each line represents the percentage of tumor area among total area of all sections from each mouse. CT26, n = 4. CT26/CXCL10, n = 5. p values were determined by two-tailed Student’s t-test. (Color figure can be accessed in the online version.)
To test whether NK cells are required for the suppression of liver metastasis of CT26/CXCL10 cells, we administered anti-asialo GM1 antibody into mice before the inoculation of CT26 and CT26/CXCL10 cells. As a result, a large number of metastatic tumor nodules was found in the liver in both groups (Fig. 5A). The liver weight (Fig. 5B) was much higher than that of antibody-untreated animals (Fig. 3D) in both groups, and there was no statistical difference between CT26- and CT26/CXCL10-inoculated groups (Fig. 5B, p = 0.375). In addition, no statistical difference was found in the proportion of tumor areas occupied in the liver sections between these groups (Fig. 5C, p = 0.523). These results suggest that NK cells play an important role in the suppression of liver metastasis of CT26/CXCL10 cells shown in Figs. 3 and 4.

In order to deplete NK cells, anti-asialo GM1 was intraperitoneally injected into mice starting from the day before tumor implantation and, thereafter, once every 5 d. CT26 and CT26/CXCL10 cells (1 × 105 cells in 50 µL PBS per mouse) were inoculated into spleens of BALB/c mice (n = 4) on day 0. (A) Livers were harvested 18 d after tumor inoculation into the spleens and the pictures were taken to compare the size of the tumors. Scale bar, 1 cm. (B) The liver weight on day 18. (C) The left lateral lobe of each liver was embedded in OCT compound. Sections from frozen blocks were stained with hematoxylin and eosin. The percentage of the area occupied by tumor among total area of all sections from each mouse was determined using ImageJ software. n = 4. Data are expressed as means ± S.D. ns, not significant. (Color figure can be accessed in the online version.)
To examine the mechanism of the inhibition of liver metastasis by CXCL10 shown in Figs. 3 and 4, we performed immunofluorescence. NKp46 is a receptor specifically expressed on mature NK cells.24) Interestingly, a large number of NKp46-positive NK cells were accumulated in the CT26/CXCL10 tumor but not in the CT26 tumor (Fig. 6A). The tumors could be discriminated from the normal liver tissues by the dense nuclear staining with DAPI. These results suggest that the forced expression of CXCL10 in the colon carcinoma cells induced NK cell infiltration into the tumor, thereby inhibiting tumor growth in the liver.

(A) Seven-micrometer frozen sections prepared from the left lateral lobe of the liver taken from the tumor-inoculated mice were fixed with acetone and stained with antibodies recognizing NKp46 or an isotype control (red), and DAPI (blue). Goat IgG was used as an isotype control. The stained sections were analyzed by fluorescence microscopy on a BZ-9000 (KEYENCE). Scale bar, 100 µm. T, tumor. The arrowheads indicate NK cells. (B) NK-92 cells that had been treated with or without anti-CXCR3 antibody were tested for their migration toward 1 to 100 ng/mL recombinant mCXCL10 (CXCL10), the supernatant of CT26 cells (CT26) or that of CT26/CXCL10 cells (CT26/CXCL10). Chemotaxis assay was performed using inserts with 8-µm pore membranes placed in the wells of a 24-well cell culture plate. Cells adhered to the lower side of the membrane filters were stained and counted in five random fields. Data are expressed as means ± S.D.
To determine if the CT26/CXCL10 cells attract NK cells, we next performed a chemotaxis assay using a human NK cell line, NK-92. As shown in Fig. 6B, recombinant murine CXCL10 induced chemotaxis of NK-92 cells in a dose-dependent manner, which was consistent with the previous report that murine CXCL10 could act on human CXCR3-expressing cells.25) The culture supernatant of CT26/CXCL10 cells induced more efficient chemotaxis than that of the CT26 cells, indicating that the CT26/CXCL10 cells secrete active CXCL10 that attracts NK cells. Pre-incubation of NK-92 cells with an antibody against CXCR3, a receptor for CXCL10,26) significantly blocked migration of these cells toward the culture supernatants of the CT26 and CT26/CXCL10 cells (Fig. 6B), indicating that the migration was dependent on the interaction between CXCR3 on NK-92 cells and its ligand secreted from the tumor cells. Together with the results in Figs. 2 and 5, it was suggested that the suppression of subcutaneous growth and liver metastasis of CT26/CXCL10 cells was likely due to the selective migration of NK cells toward the tumor sites, resulting in tumor suppression.
In this study, we examined the effects of the forced expression of CXCL10 in murine colon carcinoma CT26 cells on their growth and metastasis. We first confirmed that in vitro growth of CT26 cells was unaffected by the expression of CXCL10. In contrast, in vivo growth and tumorigenesis of CT26/CXCL10 cells was significantly suppressed after subcutaneous implantation (Fig. 1), consistent with a previous report using human melanoma cells.11) We also found that liver metastasis of CT26/CXCL10 cells was significantly suppressed compared with that of CT26 parental cells (Figs. 3, 4). Removal of NK cells by the administration of anti-asialo GM1 antibody canceled the differences in tumorigenesis between CT26 and CT26/CXCL10 cells in the skin (Fig. 2) and liver (Fig. 5), suggesting that NK cells play an important role in the rejection of CT26/CXCL10 cells. More abundant NK cells were infiltrated into the metastatic lesions of the CT26/CXCL10 cells compared with those of the CT26 cells, which was consistent with the culture supernatant of CT26/CXCL10 cells inducing chemotaxis of NK cells (Fig. 6). These results suggest that overexpression of CXCL10 in colon carcinoma cells induces NK cell-mediated anti-tumor responses.
In other reports, the possible role of CXCL10 in anti-tumor activity has been described. Overexpression of interleukins such as IL-7, IL-10 and IL-12 in the tumors induced anti-tumor activity in CXCL10 and the other CXCR3 ligand-dependent manner, and additional expression of CXCL10 resulted in the induction of better anti-tumor activity.7,27–29) CXCL10 has both anti-angiogenic activity30,31) and immune cell recruitment and activation activity, suggesting that CXCL10 suppresses tumorigenesis in the tumor microenvironment by multiple possible mechanisms. Forced expression of CXCL10 in lymphomas induced NK cell and CTL accumulation, and the augmentation of the immune response induced by IFN-γ resulted in tumor regression.32) Adoptively transferred human NK cells expressing high level of CXCR3 were selectively recruited to CXCL10-positive melanomas and suppressed their growth.33) NK cells from CXCR3-deficient mice failed to migrate to B16F10 melanoma,34) indicating that CXCR3 on NK cells plays an important role in the NK-cell-mediated suppression of melanoma growth. Similarly, the anti-tumorigenic activity of CXCL10 on colorectal cancer in the metastatic liver found in the present study is most likely due to the anti-tumor activity of NK cells that were selectively recruited to the tumor microenvironment.
In the liver metastatic model that we utilized, we did not see a difference in the weights of the spleens where CT26 and CT26/CXCL10 cells were initially injected (unpublished observation). Although the spleen functions as a reservoir of NK cells in mice,35) the tumors rapidly grew in mice that received intra-splenic injections of CT26/CXCL10 cells; i.e., the splenic weight of untreated mice was 0.1 g, whereas that of CT26- or CT26/CXCL10-injected mice was 1–2 g, respectively. In line with this notion, it was reported that liver-resident NK cells express tumor necrosis factor-related apoptosis inducing ligand (TRAIL) and efficiently suppress liver metastasis of tumor cells, whereas NK cells in the spleen rarely express TRAIL.36,37) Therefore, significant suppression of the tumor formation in the liver observed in our study (Figs. 3, 4) suggest that liver-resident NK cells were recruited into the tumor, although we were unable to exclude the possibility that NK cells were also recruited to the tumor from the blood.
The tumor microenvironment has unique characteristics compared with normal tissues, including accelerated angiogenesis and immune cell infiltration.38) Recent approaches targeting the tumor microenvironment by gene delivery systems have been demonstrated to be effective against tumor metastases that are difficult to diagnose by conventional methods. In our laboratory, we previously developed novel monoclonal antibodies against sialyl Lewis X glycans that play a critical role in tumor metastasis.39) A combination of these anti-carbohydrate antibodies and CXCL10 should be more effective against metastatic tumors compared to individual treatments.
Our study showed that forced expression of CXCL10 in mouse colon carcinomas significantly suppressed tumor progression, including metastasis. Future studies are warranted to clarify the function of CXCL10 in various other tumors and tissues in order to develop efficient therapy against tumors based on CXCL10 gene delivery.
This work was supported in part by JSPS KAKENHI under Grant Number 16H05086, Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI under Grant Number 15K14955, the Uehara Memorial Foundation, and the Hamaguchi Foundation for the Advancement of Biochemistry.
The authors declare no conflict of interest.