2019 Volume 42 Issue 1 Pages 87-93
2019 Volume 42 Issue 1 Pages 87-93
The trace element zinc is essential for the immune system, and its dysregulation and deficiency results in impaired immune function. Recent studies have shown that zinc can behave as an intracellular signaling molecule in immune cells. We have previously demonstrated that L-type calcium channel (LTCC) is involved in the regulation of zinc signaling, Zinc wave and cytokine production by stimulating Fc epsilon receptor for immunoglobulin E (IgE) in mast cells. However, it is not known whether LTCC-mediated Zinc wave is required for cytokine production by stimulation of toll-like receptors and cytokine receptors in mast cells. Here we report that stimulation of toll-like receptors and cytokine receptors can induce Zinc wave in mast cells and regulate the expression of cytokine genes. The LTCC antagonist nicardipine inhibited lipopolysaccharide (LPS)- and interleukin-33 (IL-33)-mediated Zinc wave and the induction of cytokine genes such as IL-6. Consistent with these results, the zinc chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) also inhibited LPS- and IL-33-induced cytokine gene expression. Furthermore, LPS induced Zinc wave not only in mast cells but also in dendritic cells. Together, these observations show that Zinc wave is activated by various stimuli and is linked to cytokine gene induction in immune cells.
Zinc is a nutritionally essential trace element and many diseases and psychological symptoms are associated with zinc deficiency.1,2) Many researchers have reported that zinc depletion adversely affects immune function. Natural killer cell-mediated cytotoxic activity, antibody-mediated responses, and host defenses against pathogens and tumors are all reduced in zinc-deficient mice.3,4) Approximately 10% of all genes in the human genome contains zinc-binding motifs,5) and dysregulation of zinc homeostasis is linked to a wide range of physiological defects, including the immune system.6,7)
Recent studies have revealed the presence and importance of free or labile zinc in living organisms8) and that the intracellular zinc level may change and affect cellular function and differentiation.9,10) Thus, zinc has been increasingly recognized as a potential biological signaling molecule.11–16) It is well established that synaptic zinc acts as a neurotransmitter that can mediate cell-to-cell communication.17,18) In addition to its role in intercellular communication, zinc can act as a second messenger. We have shown that stimulating the high-affinity Immunoglobulin E (IgE) receptor Fc epsilon receptor I (FcεRI) induces an increase in intracellular free zinc, and we named this phenomenon “Zinc wave.”19) Zinc wave originates from the endoplasmic reticulum (ER) and occurs within several minutes after IgE stimulation. Furthermore, we recently showed that L-type calcium channel (LTCC) on the ER membrane may act as a gatekeeper for Zinc wave that occurs when IgE mediates intracellular zinc signaling in mast cells.20)
In addition to LTCC-mediated Zinc wave by IgE stimulation in mast cells, rapid activation of intracellular zinc signaling by several stimuli for certain cellular functions has been reported.21–25) However, it is still unclear whether LTCC-mediated Zinc wave in mast cells is caused by non-IgE-mediated stimulation. In addition, LTCC-mediated Zinc wave has not been observed in immune cells other than mast cells.
Mast cells are multifunctional immune cells that participate in various biological events such as inflammation and allergy.26,27) IgE stimulation is one way to activate mast cells. It occurs when multivalent antigens cross-link antigen-specific IgE bound to FcεRI on the surface of mast cells. Cross-linked FcεRI initiates a signal transduction cascade that is dependent on the tyrosine kinase Syk.28) This activation results in the release of three classes of mediators: preformed mediators (histamine, proteases, cytokines, and enzymes), newly formed lipid mediators such as prostaglandins (PG), leukotrienes (LT), and platelet-activating factor; and newly synthesized mediators including cytokines and chemokines. A variety of IgE-independent mediators can directly trigger mast cell activation.29)
Toll-like receptors (TLRs) comprise a family of proteins that enhance the transcription of certain cytokine genes in response to various pathogenic ligands.30) TLR4 has been shown to be a receptor for lipopolysaccharide (LPS). It has already been reported that TLR4 on mast cells plays a critical role in LPS-mediated enhancement of airway eosinophilic inflammation.31) In addition, interleukin-33 (IL-33), a member of the IL-1 cytokine family, stimulates mast cells constitutively expressing IL-33R (a heterodimer of IL-1 receptor-like 1, or IL-1RL1), and various cytokines and chemokines that affects the function of other immune cells are produced. For example, it has been shown that IL-2 secreted from mast cells activated by IL-33 mediates the expansion of regulatory T cells (Treg).32) Thus, mast cells receive various stimuli and regulate immune responses including allergic responses induced by cytokines and chemokines.
In dendritic cells stimulated via TLR by 6-h exposure to LPS, the concentration of intracellular free zinc was downregulated, and cells matured faster and showed higher activation compared to untreated controls.33) Artificially depleting intracellular zinc with a zinc chelator also triggered activation and maturation of dendritic cells. This report suggested that downmodulation of basal zinc levels induces the maturation of dendritic cells. However, it is still unclear whether LTCC-mediated Zinc wave is involved in the function of dendritic cells.
Here, we report that LPS and IL-33 can induce Zinc wave in mast cells. Furthermore, we found that Zinc wave also occurs in dendritic cells. The Zinc wave can regulate cytokine gene expression by activating nuclear factor-kappaB (NF-κB) in an inhibitor of kappaB (IκB) degradation-independent manner.
C57BL/6J and BALB/c mice were obtained from Japan SLC, Inc. (Japan). The mice were maintained under specific pathogen-free conditions and those aged between 8 and 12 weeks of age were used in this study. We obtained approval from the animal research committee at Suzuka University of Medical Science for all animal experiments performed.
Reagents and AntibodiesNewport Green DCF diacetate (N7991) was purchased from Thermo Fisher Scientific (U.S.A.). Nicardipine (145-06381) and N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (340-05411) were purchased from Wako Pure Chemical Industries, Ltd. (Japan) and DOJINDO (Japan), respectively. Following antibodies were commercially obtained: Anti-IkBα (9242S) (Cell Signaling, U.S.A.), anti-phospho extracellular signal-regulated kinase (ERK)1/2 (V803A) and anti-ERK1/2 (V114A) (Promega, U.S.A.), anti-α-tubulin (T5168) (Sigma-Aldrich, U.S.A.).
Cell CultureBone marrow-derived mast cells (BMMC) were prepared as described previously.34,35) Briefly, bone-marrow cells obtained from 8-week-old C57BL6J or BALB/c mice were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 10 mU/mL penicillin, 0.1 mg/mL streptomycin and the culture supernatant of IL-3-producing Chinese hamster ovary (CHO) cells, as described previously,36) and incubated in a humidified 5% CO2 and 95% air at 37°C. After 4–5 weeks of culturing, cell-surface expression of FcεRI and c-Kit was confirmed and these cells were used for experiments (<95% mast cells).
Bone marrow-derived dendritic cells (BMDC) were generated as described previously.37) Briefly, dendritic cells (DCs) were generated from bone marrow cells with RPMI 1640 medium containing 10% fetal calf serum (FCS) in the presence of murine granulocyte macrophage colony-stimulating factor (GM-CSF) (20 ng/mL, PEPRO TECH, U.S.A.; AF-315-03) or the culture supernatant of GM-CSF-producing CHO cells, as described previously.33) Loosely adherent clustering cells were harvested on days 6–8, and CD11c+ DCs were isolated by the IMag Cell Separation System with anti-CD11c mAb-bound beads or FACSAria (BD Biosciences, U.S.A.). The sorted CD11c+ DCs were immature (CD11chigh MHC class IIlow), and the purity was >95% (data not shown).
Flow CytometryThe intracellular zinc was measured by flow cytometry. Briefly, BMMCs were incubated with 2.0 µM Newport Green for 30 min at 37°C, and the cells were stimulated with LPS (Sigma-Aldrich, L2637) or IL-33 (R&D system, 3636-ML) at 37°C. After stimulation, the fluorescent signals were detected using FACSCalibur™ flow cytometer.
Measurement of Cytokines and Chemical MediatorsCells were activated as described above, and IL-6 in culture supernatants was measured using an enzyme-linked immunosorbent assay (ELISA) kit (BD, 555240).
Real-Time PCR AnalysisCells were homogenized with Sepasol RNAI (Nacalai Tesque, Japan; 09379-55), and total RNA was isolated following the manufacturer’s instructions. For standard RT-PCR, cDNA was synthesized from 1 µg of total RNA with reverse transcriptase (ReverTra Ace; Toyobo, Japan; TRT-101) and 500 ng of oligo (dT) primer (Life Technologies Corporation, U.S.A.; 58862) for 30 min at 42°C. A portion of the cDNA was used for real-time PCR. The relative expression of Il-6 gene was determined compared to a reference gene g3pdh using the SYBR® Green reagent (TaKaRa, Japan; RR820A). Primers used in these experiments were purchased from TaKaRa, and the sequences were as follows: IL-6: forward primer, 5′-GAG GAT ACC ACT CCC AAC AGA CC-3′ and reverse primer, 5′-AAG TGC ATC ATC GTT GTT CAT ACA-3′; glycerol-3-phosphate dehydrogenase (G3PDH): forward primer, 5′-TTC ACC ACC ATG GAG AAG GCC G-3′ and reverse primer, 5′-GGC ATG GAC TGT GGT CAT GA-3′.
Luciferase Reporter AssayThe pGL4/NF-κB promoter vector and pRL-TK vector were transiently co-transfected into mast cells using a two-step electroporator NEPA21 (Nepa Gene, Japan). Cells were harvested 24 h after transfection and stimulated with dinitrophenylated human serum albumin (DNP-HSA) for 6 h. Luciferase activity of the total cell lysates was measured using the Dual-Luciferase Reporter Assay System (Promega, E1910).
Cell Lysates and ImmunoblottingBMMCs were harvested, lysed in lysis buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, proteinase inhibitors, 5 µg/mL pepstatin, 10 µg/mL leupeptin) for 30 min at 4°C, and spun at 12000 × g at 4°C for 30 min. The eluted and reduced samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 5–20% gradient polyacrylamide gel (Wako, 197-15011) and transferred to a PVDF membrane (Immobilon-P, Millipore, U.S.A.; IPVH00010). For immunoblotting, the membranes were incubated with primary antibodies for 1 h at room temperature. The membranes were then incubated with HRP-conjugated anti-mouse (Thermo Fisher Scientific, 62-6520) or anti-rabbit (Thermo Fisher Scientific, 65-6120) antibody for 1 h at room temperature. After extensive washing of the membranes, immunoreactive proteins were visualized using the Western Lightning-ECL system (GE-Healthcare, U.S.A.; RPN2232) according to the manufacturer’s recommendation. The PVDF membranes were exposed to Fuji RX film (FUJIFILM, Japan; RX-U). Densitometric analysis was performed using an LAS-4000 fluorescence image analyzer (FUJIFILM).
Statistical AnalysisData were statistically analyzed using Students’ two-tailed t-test. Additional statistically analyzed were done by Tukey–Kramer multiple comparison test. Data were considered statistically significant when the p value was less than 0.05.
We have previously shown that IgE stimulation induces Zinc wave in mast cells and that Zinc wave is regulated by LTCCs on the membrane of the ER.20) In this study, we first investigated whether the level of intracellular free zinc changes in response to LPS and IL-33. We examined the changes in the intracellular zinc level using the zinc indicator Newport Green DCF. As shown in Fig. 1A, approximately 1.5-fold increase in intracellular zinc was observed at 15 min after stimulation, and the zinc level significantly decreased at 60 min after stimulation. The LTCC antagonist nicardipine completely inhibited this induction (Fig. 1B). Similarly, stimulating mast cells with LPS and IL-33 for 30 min also induced Zinc wave, and this induction was significantly suppressed by nicardipine treatment (Figs. 1C, D). These data indicated that Zinc wave is induced by various stimuli including LPS and a cytokine.

(A)–(D) The intracellular zinc level after 0–60 min (A) or 15 min (B) stimulation with 10 ng/mL DNP-HSA, 30 min stimulation with 0.1 µg/mL LPS (C) and 30 min stimulation with 10 ng/mL IL-33 (D) was examined using the fluorescent zinc indicator Newport Green in mast cells with or without pre-treatment with 100 µM nicardipine for 30 min (B)–(D). Data represent the relative fluorescence intensity of Newport Green. Data show mean + standard deviation (S.D.). *** p < 0.001, two-tailed Student’s t-test.
In mast cells, IgE, LPS, and IL-33 stimulation can induce the production of cytokines such as IL-6 and tumor necrosis factor-alpha (TNF-α), and these cytokines are involved in inflammatory and allergic responses. To determine whether LTCC-mediated Zinc wave affects these mast cell-activating events, we investigated the effect of nicardipine treatment on IgE-, LPS-, and IL-33-mediated cytokine production and gene induction in mast cells. Inhibition of Zinc wave by nicardipine reduced the IgE-mediated IL-6 production in a dose-dependent manner (Fig. 2A). In addition, nicaridpine or TPEN treatment at the concentrations used in this study did not affect the viability of mast cells (data not shown). Furthermore, we examined the role of zinc in cytokine production in mast cells using the zinc chelator TPEN. As shown in Fig. 2D, IgE-mediated cytokine production was significantly reduced in TPEN-treated mast cells, suggesting that zinc is necessary in this process. Similar results were obtained in LPS- and IL-33-induced cytokine production (Figs. 2B, C, E, F). Taken together, these results showed that Zinc wave is involved in IgE-, LPS- and IL-33-mediated cytokine production in mast cells.

(A)–(C) IL-6 production after 1 h stimulation with 10 ng/mL DNP-HSA (A), 6 h with 1 µg/mL LPS (B) and 12 h with 1 ng/mL IL-33 (C) in mast cells with or without pretreatment with 10 µM nicardipine for 30 min were determined by ELISA. (D)–(F) IL-6 production after 1 h stimulation with 10 ng/mL DNP-HSA (D), 6 h with 1 µg/mL LPS (E) and 12 h with 1 ng/mL IL-33 (F) in mast cells with or without pretreatment with 10 µM TPEN for 30 min were determined by ELISA. Data show mean + S.D. * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA with Tukey–Kramer multiple comparison test (A)–(C) or two-tailed Student’s t-test (D)–(F).
To elucidate the molecular mechanism(s) underlying defective cytokine production in nicardipine-treated mast cells, we analyzed the mRNA levels of cytokines by quantitative PCR. IgE stimulation of mast cells led to an increase in the Il6 mRNA level (Fig. 3A), and treating these cells with nicardipine decreased the Il6 mRNA level in a dose-dependent manner (Fig. 3A). Furthermore, the zinc chelator TPEN significantly inhibited the IgE-mediated expression of Il6 (Fig. 3D). We next tested whether LPS- and IL-33-mediated Zinc wave is involved in the induction of cytokine gene expression. As shown in Figs. 3B and C, nicardipine significantly reduced LPS- and IL-33-mediated upregulation of Il6 mRNA in a dose-dependent manner. Furthermore, we confirmed that zinc is involved in the LPS- and IL-33-mediated induction of cytokine genes such as Il6 (Figs. 3E, F). Thus, LTCC-mediated Zinc wave is involved in IgE-, LPS- and IL-33-induced transcription of Il6 gene.

(A)–(C) IL-6 gene transcription after 1 h stimulation with 10 ng/mL DNP-HSA (A), 1 µg/mL LPS (B) and 1 ng/mL IL-33 (C) in mast cells with or without pretreatment with 1, 10, or 100 µM nicardipine for 30 min were determined by quantitative RT-PCR. (D)–(F) IL-6 gene transcription after 1 h stimulation with 10 ng/mL DNP-HSA (D), 1 µg/mL LPS (E) and 1 ng/mL IL-33 (F) in mast cells with or without pretreatment with 10 µM TPEN for 30 min were determined by quantitative RT-PCR.Data show mean + S.D. * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA with Tukey–Kramer multiple comparison test (A)–(C) or two-tailed Student’s t-test (D)–(F).
We next investigated whether Zinc wave is also involved in LPS-mediated cytokine production in dendritic cells. As shown in Fig. 4A, LPS triggered Zinc wave in dendritic cells. However, the increase in the Newport Green signal was suppressed almost to the basal level two hours after treating the cells with nicardipine (Fig. 4A). Consistent with this result, LPS-induced cytokine production was also suppressed in nicardipine-treated dendritic cells (Fig. 4B). Treatment with TPEN also reduced IL-6 production, indicating the involvement of zinc in LPS-mediated cytokine production (Fig. 4B). In addition, LPS-mediated gene expression of Il6 was decreased in nicardipine- or TPEN-treated dendritic cells (Fig. 4C). These results suggest that Zinc wave is involved in IL-6 production in dendritic cells.

(A) The level of intracellular zinc 2 h after stimulating dendritic cells with 1 µg/mL LPS was examined using the fluorescent Zn indicator Newport Green in dendritic cells with or without pretreatment with 10 µM nicardipine for 30 min. The bar graph shows the relative fluorescent intensity of Newport Green. (B) IL-6 production 6 h after stimulating dendritic cells with 1 µg/mL LPS with or without pretreatment with 10 µM Nicardipine or 0.1 µM TPEN for 30 min, determined by ELISA. (C) The level of IL-6 transcription upon stimulating dendritic cells with 1 µg/mL LPS for 3 h with or without pretreatment with 10 µM nicardipine or 0.1 µM TPEN for 30 min, determined by quantitative RT-PCR. Data show mean + S.D. * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA with Tukey–Kramer multiple comparison test or two-tailed Student’s t-test.
Since NF-κB pathway is required for the regulation of the expression of inflammatory cytokines such as IL-6 and TNF-α in mast cells,36,38,39) we investigated whether LTCC-mediated Zinc wave is required for NF-κB activation. We performed a luciferase reporter assay using mast cells in which NF-κB reporter gene was introduced. As shown in Fig. 5A, IgE-mediated reporter activity of NF-κB was significantly inhibited in nicardipine-treated mast cells, suggesting that LTCC-mediated Zinc wave regulates the activation of NF-κB pathway. Next, we examined the effect of nicardipine on the degradation of IκBα that regulates the nuclear localization of NF-κB. In mast cells, IgE stimulation induced the degradation of IκB and the activation of MAPK in a time-dependent manner (Fig. 5B, C). However, inhibiting Zinc wave with nicardipine did not affect IgE-induced IκB degradation in mast cells (Figs. 5B, D). These results suggested that Zinc wave is involved in IgE-mediated NF-κB signaling pathway in an IκB-independent manner.
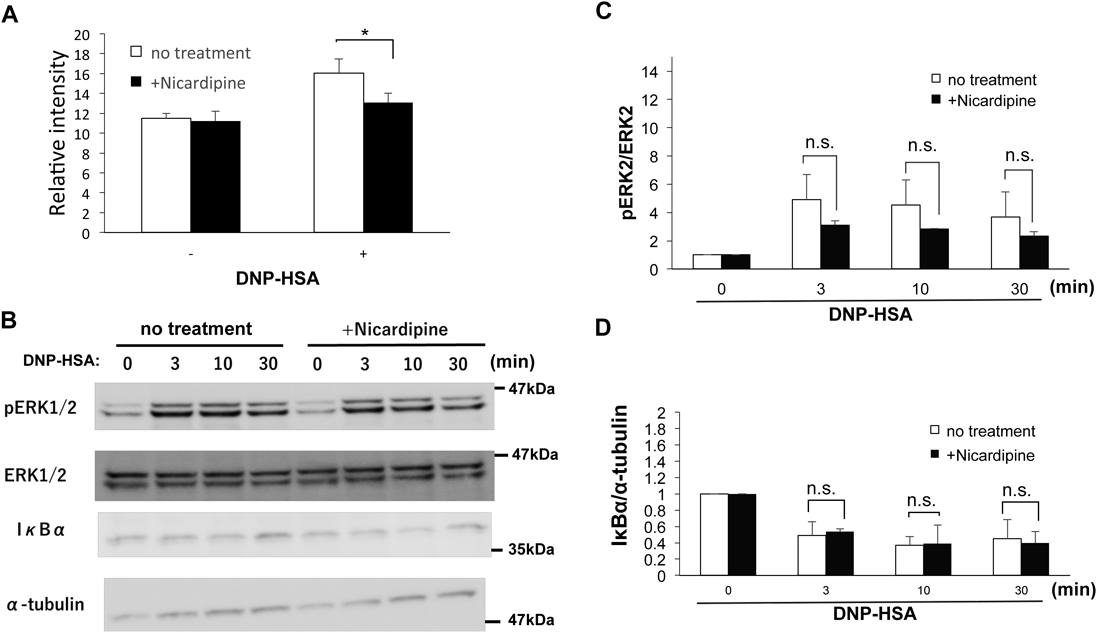
(A) NF-κB activity in mast cells stimulated via IgE with or without nicardipine treatment, determined by the luciferase reporter assay. Mast cells were electroporated with plasmids NF-κB/Luc and phRL-TK. Results were normalized to the Renilla luciferase activity. (B)–(D) ERK1/2 phosphorylation and IκBα degradation. After antigenic stimulation, cells were lysed and the cytosol fraction was immunoblotted with anti-phospho-ERK or anti-IκBα antibody. Data were normalized to the expression levels of non-phosphorylated ERK and α-tubulin. Data show mean + S.D. * p < 0.05, two-tailed Student’s t-test. N.S., not significant.
Recent studies have shown that zinc can behave as an intracellular signaling molecule and that increased intracellular free zinc is involved in the activation of variety of cells including mast cells, primary monocytes, and granulocytes.40–42) We have previously demonstrated that mast cells stimulated via IgE stimulation rapidly release zinc from the ER into the cytoplasmic environment within minutes after activation, and we named this phenomenon “Zinc wave.”19) In addition, we reported that LTCC on the membrane of ER acts as the gatekeeper for Zinc wave.14,20) Although increased free intracellular zinc was observed in other immune cells activated via various stimuli, we have only reported the activation of LTCC-mediated Zinc wave in response to IgE-mediated mast cell stimulation. In this study, we showed that LPS and IL-33 also trigger LTCC-mediated Zinc wave in mast cells and dendritic cells.
It has been shown by our collaborators that stimulating DCs with LPS affects the expression of zinc transporter genes ZIP-6, ZIP-10, ZnT-1, ZnT-4, and ZnT-6 in dendritic cells and leads to decrease in intracellular zinc levels.33) This is a process required in the LPS-mediated upregulation of MHC class II surface expression. In this study, we showed that stimulating cells with LPS triggers Zinc wave in dendritic cells. In addition to this, we showed that LTCC is involved in this process, as the increase in intracellular free zinc was abrogated by treating the cells with the LTCC antagonist nicardipine. Therefore, the increase in intracellular free zinc triggered by LPS can be regulated by LTCC like in mast cells.
Our data showed that LTCC-mediated Zinc wave is involved in the regulation of cytokine gene expression. Nicardipine can inhibit IgE-, LPS-, and IL-33-mediated induction of IL-6. In addition, IgE-, LPS-, and IL-33-induced cytokine production was suppressed by nicardipine in mast cells and dendritic cells. Furthermore, we showed that the zinc chelator TPEN inhibited IgE-, LPS- and IL-33-mediated cytokine gene induction. These results indicated that the upregulation of intracellular free zinc can regulate the expression of cytokine genes in mast cells stimulated via IgE or by LPS and IL-33.
How might these intracellular free zinc cause the upregulation of gene expression? In general, transcription of IL-6 and TNF-α is regulated by the transcription factor NF-κB. Zinc wave is likely to be involved in the IgE-induced NF-κB-signaling pathway. In this study, we showed that the LTCC antagonist nicardipine inhibits the IgE-induced reporter activity of NF-κB, which indicated that LTCC-mediated Zinc wave affects the IgE-induced NF-κB-signaling pathway. Furthermore, we showed that IgE-induced IκB degradation was not inhibited in nicardipine-treated mast cells, indicating that free zinc is not involved in IgE-mediated IκB degradation. NF-κB-mediated transactivation can be divided into the following three steps. First, NF-κB dissociates from IκB after IκB is phosphorylated and degraded. It is then translocated from the cytosol to the nucleus and finally binds to its targets.43) Hence, it raises the possibility that Zinc wave promotes NF-κB translocation thereby enhancing the binding of NF-κB to the target sites. This idea is supported by the report that NF-κB requires zinc ion for optimally binding to DNA.44–47)
In summary, we showed that Zinc wave is involved in IgE-, LPS- and IL-33-mediated signaling pathways. In addition, LTCC-mediated Zinc wave may function as a positive regulator of inflammatory cytokines in a manner independent of IκB degradation. Thus, Zinc wave has diverse functions in the regulation of cytokines in various immune cells other than mast cells. These findings will help us understand the regulation and importance of intracellular Zinc signaling during immune response.
We thank Dr. M. Kato for critical reading. This work was supported in part by JSPS KAKENHI Grant Numbers JP16K15152 and JP18H05299 (K.N.), and SENSHIN Medical Research Foundation (K.N.). This work was partially supported by Grants-in-Aid for Scientific Research (C) (15K08416) from the Japan Society for the Promotion of Science (JSPS), the Platform Project for Supporting Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and the Tojuro Iijima Foundation for Food Science and Technology. R.U. was supported by Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan, and Public Interest Incorporated Foundation Tsukushi Scholarship Research Fund.
The authors declare no conflict of interest.