2019 Volume 42 Issue 2 Pages 149-157
2019 Volume 42 Issue 2 Pages 149-157
To date, limited drug information is available for the individual optimization of pharmacotherapy. The author attempted multiple evaluations of patient data on factors related to the pharmacokinetics, drug efficacy, and adverse reactions observed in clinical settings. Through the clinical studies, drug information on the individual optimization of pharmacotherapy needed by health professionals including physicians and pharmacists was identified. Major findings were: 1) Cachectic cancer patients had high plasma concentrations of oxycodone via the reduction of CYP3A activity. The metabolic reduction in cachectic cancer patients was potentially related to the elevated serum level of interleukin-6. 2) Dopamine receptor D2 (DRD2) genetic mutations and being female led to poor antiemetic efficacy of the treatment of opioid-induced nausea in prochlorperazine-treated patients. The opioid receptor μ1 (OPRM1) wild genotype in addition to being female and having high plasma concentrations of prochlorperazine increased prolactin secretion during oxycodone treatment. 3) Rheumatoid arthritis patients with a genetic mutation of ATP-binding cassette subfamily B member 1 (ABCB1) had high plasma concentrations of tacrolimus and its 13-O-demethylate. The ABCB1 genetic mutation and associated high plasma concentration of tacrolimus decreased kidney function. 4) Chronic inflammation increased the plasma voriconazole concentration via its poor metabolism, whereas it did not alter the plasma itraconazole concentration. Although co-administration of prednisolone did not affect the plasma concentration of triazole antifungals, it weakly increased voriconazole metabolism. 5) In breastfeeding women, the median milk/plasma concentration ratio of amlodipine was 0.85. However, the observed relative infant dose of amlodipine in most patients was less than 10%.
Patient data observed in clinical settings are diverse and complex. Multiple evaluations of patient data including host factors (gender, age, pregnancy, obesity, hepatic and renal function, etc.), environmental factors (concomitant drugs, supplements, food, alcohol consumption, smoking, etc.), disease factors (cancer, cachexia, inflammation, diabetes mellitus, etc.), and genetic factors (genetic variants and epigenetic factors) and their appropriate applications to each patient are needed for the individual optimization of pharmacotherapy1,2) (Fig. 1). To date, limited clinical evidence and drug information are available for the individual optimization of pharmacotherapy. The appropriateness of the clinical evidence and uniform drug information for analyzing diverse and complex individual patient data has not been fully evaluated and validated in clinical situations. The author attempted multiple evaluations of patient factors related to pharmacokinetic disposition, drug efficacy, and adverse reactions observed in clinical settings. Additionally, drug information required by health professionals including physicians and pharmacists on the proper use of pharmaceuticals was obtained in clinical settings. Through the clinical research in collaboration with physicians, the author provided drug information on the individual optimization of pharmacotherapy for health professionals.

Our research has mainly addressed the challenges of cancer pain relief in palliative care,3–8) immunosuppressive therapy for autoimmune diseases,9,10) antimicrobial therapy for serious conditions,11,12) and pharmacotherapy during the perinatal period.13–15) In the present review, the author describes five achievements in recent clinical research on patient factors associated with pharmacokinetics, drug efficacy, and adverse reactions as well as information on the appropriate use of pharmaceuticals. The clinical studies reviewed were conducted in accordance with the principles of the Declaration of Helsinki and its amendments and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The Ethics Committee of Hamamatsu University School of Medicine approved the study protocols.
Oral oxycodone is converted primarily to inactive noroxycodone by hepatic CYP3A, while CYP2D6 participates in oxycodone conversion to oxymorphone.16) Cancer patients show great variability in oxycodone pharmacokinetics.17) Our previous study revealed that genetic polymorphisms of CYP2D6 and CYP3A5 have no effects on oxycodone pharmacokinetics.3) Generally, most cachectic cancer patients receive opioid analgesics such as morphine and oxycodone for severe cancer pain relief in clinical situations. In cachectic conditions, proinflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α are secreted into the circulating blood.18) The serum levels of proinflammatory cytokines are inversely related to hepatic CYP3A-associated drug elimination.19) Elevated proinflammatory cytokine levels are potentially responsible for a large variation in plasma oxycodone exposure in cancer patients. Cachectic cancer patients occasionally exhibit central nervous system symptoms such as depression, sleepiness, and somnolence.20,21) These symptoms may be related to treatment with central nervous system depressants such as opioid analgesics or to increased proinflammatory cytokines in the blood. Our study investigated the associations between plasma oxycodone, central symptoms, cachexia stage, and serum levels of proinflammatory cytokines in patients with cancer pain.
The observational study (UMIN000020723) was conducted at Hamamatsu University Hospital (Hamamatsu, Japan). Forty-seven Japanese patients receiving oxycodone extended-release tablets (OxyContin, Shionogi & Co., Ltd., Osaka, Japan) twice a day for severe cancer pain were enrolled. The patients had advanced cancer stages according to the tumor-node-metastasis staging classification. The degree of cachexia was classified into three stages, precachexia, cachexia, and refractory cachexia, using clinical symptom-based diagnostic criteria.22) The numbers of patients with precachexia, cachexia, and refractory cachexia were 10, 24, and 13, respectively. The serum level of C-reactive protein (CRP) was significantly higher in cancer patients with refractory cachexia and cachexia than in those with precachexia (p < 0.001 and p < 0.001, respectively). Cancer patients with refractory cachexia and cachexia had higher plasma concentrations of oxycodone than those with precachexia (p < 0.01 and p = 0.04, respectively). Cancer patients with cachexia had a lower noroxycodone metabolic ratio than those with precachexia (p = 0.04). The serum IL-6 level was significantly higher in cancer patients with cachexia and refractory cachexia than in those with precachexia (p < 0.01 and p < 0.01, respectively), although the serum levels of IL-1β and tumor necrosis factor-α did not differ. The serum IL-6 level was positively associated with the plasma oxycodone concentration (r = 0.49, p < 0.01) and negatively associated with the metabolic ratio to noroxycodone (r=−0.35, p = 0.02) (Fig. 2). The plasma oxycodone concentration was not related to the incidence of somnolence. The incidence of somnolence was associated with the cachexia stage and serum IL-6 level (p < 0.01 and p < 0.01, respectively).

(a) Plasma oxycodone, (b) metabolic ratio to noroxycodone, and (c) metabolic ratio to oxymorphone. The correlations were analyzed using Pearson’s correlation coefficient.
In summary, the progression of cancer cachexia increased the plasma oxycodone concentration via the reduction of hepatic CYP3A activity. The metabolic decrease in cancer patients with higher cachexia stage was related to the elevated serum level of IL-6. The symptom of somnolence was observed in cachectic cancer patients with higher plasma oxycodone and higher serum IL-6 levels.
Patients treated with opioid analgesics frequently experience adverse drug reactions leading to nonadherence.23,24) Digestive symptoms such as nausea and vomiting are adverse reactions commonly observed early after the introduction of opioid analgesics.24,25) Opioid-induced nausea and vomiting (OINV) are caused by direct stimulation of the chemoreceptor trigger zone, enhancement of vestibular sensitivity, and reduction of gastrointestinal motility.26) Prochlorperazine (PCZ), a dopamine receptor D2 (DRD2) antagonist, is routinely prescribed for OINV prophylaxis in Japan.27) PCZ efficacy and adverse reactions exhibit interindividual variations during treatment with opioid analgesics. In contrast, PCZ enhances prolactin secretion into the circulating blood through the inhibition of dopaminergic systems in the anterior lobe of the pituitary gland.28,29) The hypothalamus controls the serum prolactin level via dopamine secretion.30) Opioid analgesics bind to opioid receptor μ1 (OPRM1) expressed on the hypothalamus and inhibit the dopaminergic systems.31,32) A few significant genetic mutations of DRD2 and OPRM1 have been found in humans.33,34) The associations between DRD2 and OPRM1 genotypes, antiemetic efficacy of PCZ, and serum prolactin elevation have not been fully evaluated during treatment with opioid analgesics. This study investigated the impacts of nongenetic and genetic factors on clinical responses to PCZ in cancer patients receiving oral oxycodone.
This observational study (UMIN000011323) was performed at Hamamatsu University Hospital. A total of 70 Japanese patients treated with oral PCZ tablets (Novamin, Shionogi & Co., Ltd.) three times daily for OINV prophylaxis were enrolled. All received oral PCZ tablets concomitantly with oxycodone tablets for severe cancer pain. The antiemetic efficacy of PCZ for OINV was observed for 14 d after starting PCZ treatment. The oxycodone dose and plasma PCZ concentration were not related to the incidence of OINV. Patients with the DRD2 TaqIA A1A2 + A1A1 alleles and with the DRD2 TaqIB B1B2 + B1B1 alleles had a higher incidence of nausea than those with the A2A2 allele and the B2B2 allele, respectively (Table 1). A higher incidence of vomiting was observed in women than in men. Women had higher serum levels of prolactin than men before and after PCZ administration (p < 0.01 and p < 0.01, respectively). The serum prolactin level was mutually related to plasma PCZ concentration (ρ = 0.24, p = 0.04). Serum levels of prolactin were significantly higher in patients with the OPRM1 118AA allele than in those with the 118AG + 118GG alleles (p < 0.01), while the levels were not associated with the DRD2 genotypes (Fig. 3).
| n | Nausea | Vomiting | ||||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | p | n | (%) | p | |||
| Gender | ||||||||
| Male | 45 | 14 | 31 | 0.31 | 3 | 7 | < 0.01 | |
| Female | 25 | 11 | 44 | 9 | 36 | |||
| OPRM1 | ||||||||
| A118G | AA | 25 | 11 | 44 | 0.31 | 5 | 20 | 0.74 |
| AG + GG | 45 | 14 | 31 | 7 | 16 | |||
| DRD2 | ||||||||
| TaqIA | A2/A2 | 29 | 6 | 21 | 0.04 | 2 | 7 | 0.11 |
| A1/A2 + A1/A1 | 41 | 19 | 46 | 10 | 24 | |||
| TaqIB | B2/B2 | 29 | 6 | 21 | 0.04 | 2 | 7 | 0.11 |
| B1/B2 + B1/B1 | 41 | 19 | 46 | 10 | 24 | |||
Fisher’s exact test.

(a) DRD2 TaqIA, (b) DRD2 TaqIB, and (c) OPRM1 A118G. Box plots represent the median levels with 25th and 75th percentiles. The effects of each genotype were analyzed using the Mann–Whitney U-test, † p < 0.01.
Our subsequent research including CYP genotype analyses showed that homozygotes for CYP3A5*3 alleles tended to increase the plasma concentration of PCZ and its active metabolite in cancer patients.8) In summary (Fig. 4), being female and having the DRD2 TaqIA and TaqIB mutations attenuated the antiemetic efficacy of PCZ for OINV. Additionally, the OPRM1 wild genotype, in addition to being female and high plasma PCZ exposure, increased the serum prolactin level. These observations indicate that genetic polymorphisms of DRD2 and OPRM1 are responsible for the large interindividual variations in PCZ responses during oxycodone treatment.

Rheumatoid arthritis (RA) is caused by T cell-mediated inflammation in the synovial membrane of the joints.35) Tacrolimus is a T cell-targeted immunosuppressive drug for RA treatment.36) In organ transplantation, a large variation in blood tacrolimus exposure was observed among recipients.37) Tacrolimus administration occasionally causes blood exposure-associated adverse reactions such as infectious complications, nephrotoxicity, and cardiotoxicity.38) Oral tacrolimus is principally eliminated by the hepatic CYP3A4 and 3A5 metabolism.39) In organ transplant recipients, the homozygotes for the CYP3A5*3 alleles require a lower dose of tacrolimus to reach the target range of blood concentration than the *1 allele carriers.40–43) Tacrolimus is an intestinal P-glycoprotein substrate, and its gene is encoded by the ATP-binding cassette subfamily B member 1 (ABCB1).44) Some studies in organ transplant recipients demonstrated poor associations between the genetic polymorphisms of ABCB1 and blood tacrolimus exposure.40,41,45) There is still controversy about whether ABCB1 genotypes contribute to the pharmacokinetic variability of tacrolimus in organ transplant recipients.42,43) To date, few significant factors determining tacrolimus pharmacokinetics and clinical responses have been found in RA patients. This study investigated the impacts of CYP3A5 and ABCB1 genetic mutations on blood tacrolimus exposure and metabolism in RA patients. It also evaluated the contributions of their genetic polymorphisms to tacrolimus efficacy and kidney function.
This observational study was performed at Hamamatsu University Hospital. Seventy Japanese RA patients initiating oral tacrolimus capsule (Prograf, Astellas Pharma, Tokyo, Japan) treatment once a day in the evening were enrolled. In this study, the incidence of drug resistance or intolerance within 28 d after starting the medication was considered to indicate tacrolimus discontinuation. Sixty-four of 70 RA patients achieved disease remission with tacrolimus dose titration. Patients with the homozygous CYP3A5*3 allele had a higher dose-adjusted blood concentration of tacrolimus than those with the *1 allele (p = 0.03). The 13-O-demethylate metabolic ratio in patients with the homozygous CYP3A5*3 allele was lower than that in patients with the CYP3A5*1 allele (p < 0.01). Higher dose-adjusted blood concentrations of tacrolimus and its 13-O-demethylate were observed in patients with the homozygous ABCB1 3435T allele (p = 0.03 and p = 0.03, respectively). The absolute blood concentration of tacrolimus was inversely correlated with the estimated glomerular filtration rate (eGFR), while it was not associated with the serum CRP level (Fig. 5). The presence of the homozygous ABCB1 3435T allele was associated with a decreased eGFR, while the CYP3A5 genotype was not (Fig. 6). Six RA patients underwent discontinuation within 4 weeks after starting the medication because of a poor response to tacrolimus. A higher incidence of tacrolimus discontinuation was observed in patients carrying the homozygous CYP3A5*1 than carrying the homozygous *3 allele (odds ratio, 61.5, p < 0.01). The ABCB1 genotype was not associated with the incidence of oral tacrolimus discontinuation among patients enrolled.

(a) Estimated glomerular filtration rate (eGFR) and (b) C-reactive protein (CRP). The correlations were analyzed using Pearson’s correlation coefficient.

(a) CYP3A5*3 and (b) ABCB1 C3435T. eGFR, estimated glomerular filtration rate. Box plots represent the median level (bold line) with 25th and 75th percentiles. The effects of each genotype were analyzed using the Mann–Whitney U-test or Kruskal–Wallis test with post-hoc Bonferroni comparison. In multivariate analysis, ABCB1 3435TT resulted in a slight decline in eGFR (standardized partial regression coefficient = −0.25, p = 0.05).
In summary, CYP3A5*3 was associated with a high blood concentration of tacrolimus due to poor metabolism. In contrast, ABCB1 C3435T raised blood concentrations of tacrolimus and its 13-O-demethylate. These observations indicate that the ABCB1 genetic mutation and its associated higher blood exposure of tacrolimus are involved in the deterioration of kidney function in RA patients.
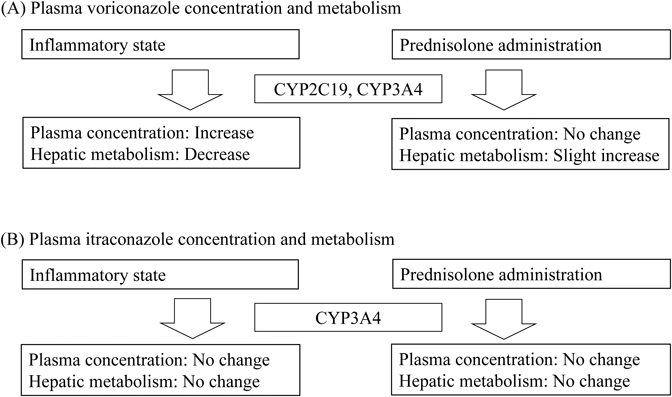
Voriconazole (VRCZ) is a second-generation broad-spectrum triazole antifungal agent with potent activity against Candida, Cryptococcus, and Aspergillus species.46) VRCZ is mainly used for the treatment of serious and invasive fungal infections, which are occasionally observed during immunosuppressant therapy.47) A nonlinear pharmacokinetic profile of VRCZ was observed in clinical situations.48,49) CYP2C19 and CYP3A4 are important CYP enzymes involved in VRCZ elimination.50) Itraconazole (ITCZ) is also a triazole antifungal agent with potent activity against systemic and superficial mycoses including Candida and Aspergillus species.51) Hepatic CYP3A4 is the major CYP enzyme responsible for ITCZ metabolosm.52) Generally, most immunocompromised patients receiving immunosuppressant therapy also receive a triazole antifungal for prophylaxis or for the treatment of fungal infections, and the alterations in CYP3A4 activity may influence drug elimination.50,53) Immunocompromised patients are at risk for infectious and inflammatory diseases. Inflammation and its associated secretion of proinflammatory cytokines into the circulating blood are related to decreased CYP3A4 expression.19) Additionally, most patients receiving a triazole antifungal concomitantly receive glucocorticoids including prednisolone and methylprednisolone. Glucocorticoids are characterized as potential inducers of CYP enzymes such as CYP2C19 and CYP3A4.54,55) However, few clinical studies have evaluated the impact of concomitant glucocorticoid administration on plasma concentrations of triazole antifungals. This study investigated the pharmacokinetic characteristics of triazole antifungals under the conditions of inflammation and concomitant glucocorticoid administration.
The observational study was performed at Hamamatsu University Hospital. Forty-one Japanese patients receiving VRCZ (Vfend tablet and injection, Pfizer Japan Inc., Tokyo, Japan) and 42 patients receiving ITCZ (Itrizole oral solution, Janssen Pharmaceutical K.K., Tokyo, Japan) for prophylaxis or treatment of an invasive fungal infection were enrolled. Thirty-five patients (10 in the VRCZ treatment group and 25 in the ITCZ treatment group) were co-treated with oral prednisolone (Predonine, Shionogi & Co., Ltd.). No correlation was observed between the plasma concentrations of VRCZ and its N-oxide. The serum level of CRP was positively correlated with the dose-adjusted plasma concentration of VRCZ (r = 0.61, p < 0.01), while it was negatively correlated with the metabolic ratio of VRCZ N-oxide (r=−0.52, p < 0.01) (Fig. 7). In glucocorticoid co-treated patients, a higher daily dose of prednisolone led to a higher metabolic ratio of VRCZ N-oxide (r = 0.72, p = 0.02). A positive correlation was observed between the plasma concentration of ITCZ and its hydroxide (r = 0.89, p < 0.01). There were no associations between plasma ITCZ exposure and metabolism and serum CRP level or prednisolone daily dose.

(a) Plasma VRCZ and (b) its metabolic ratio to N-oxide. CRP, C-reactive protein. The correlations were analyzed using Pearson’s correlation coefficient.
In summary (Fig. 8), chronic inflammation increased the plasma VRCZ concentration via its poor metabolism, whereas it did not alter the plasma ITCZ exposure. Although co-administration of prednisolone did not alter the plasma concentration of triazole antifungals, it weakly increased VRCZ metabolism.
Postpartum women with pregnancy-induced hypertension (PIH) commonly receive long-acting dihydropyridine calcium channel blockers including amlodipine (AML) and extended-release nifedipine.56,57) According to the U.S. National Library of Medicine Drugs and Lactation Database,58) more data on safety are required for the clinical administration of AML to lactating women. However, no adverse reactions in breastfed infants have been reported with the empirical maternal use of AML for PIH while breastfeeding.59–61) Few well-designed clinical studies have been conducted on AML transfer to maternal breast milk. Oral AML is largely absorbed from the intestine, mainly metabolized by hepatic CYP3A4,62) and slowly eliminated with a long plasma half-life.63) Additionally, pregnancy and its related hormones increase hepatic CYP3A4 activity.64,65) However, the plasma disposition of AML has not been fully characterized in lactating hypertensive women.
Determination of the relative infant dose (RID) is one useful method for estimating breastfed infant risk.66) When the RID is less than 10%, the medication is generally considered safe while breastfeeding because of unlikely adverse reactions in infants.66) In one case report, AML in infant plasma samples was not found after breastfeeding for 4 d.59) There have been few clinical reports on the RID of AML. Breastfeeding has many health benefits for both mothers and their infants. Medication to treat hypertension during pregnancy and after delivery is required from the viewpoint of preventing maternal cerebrovascular disorders. We performed a study to determine the plasma disposition of AML and its transfer to maternal breast milk in PIH and estimated the breastfed infant risk in clinical settings.
The observational study (UMIN000013632) was performed at Hamamatsu University Hospital and enrolled 31 Japanese patients starting oral AML besilate tablets (Sawai Pharmaceutical Co., Ltd., Osaka, Japan) for severe PIH after delivery. The AML dose was titrated according to the systolic/diastolic blood pressure goal of less than 140/90 mmHg. The RID (%) was estimated from the following equation: (Cmilk × Vmilk/Dmaternal) × 100%, where Cmilk is the AML concentration in milk, Vmilk the daily volume of milk intake by infants, and Dmaternal the maternal body weight-adjusted AML daily dose.66) The daily volume of milk intake by newborns was assumed to be 150 mL per kg.67) The enrolled women had a median systolic/diastolic blood pressure of 152/94 mmHg before starting the medication and subsequently received a mean maternal dose of 6.0 mg of AML. Maternal blood and milk samples were collected just before dosing on day 10 (interquartile range [IQR], days 8–10) after starting AML. The median plasma concentration of AML was 15.5, while the milk concentration was 11.5 ng/mL (Fig. 9). There were large variations in the AML dose-adjusted plasma (IQR, 135–209 ng/mL per mg/kg) and milk (96.7–205 ng/mL per mg/kg) concentrations in lactating women. The median milk/plasma concentration ratio of AML was 0.85 (IQR, 0.74–1.08) (Fig. 10). The median infant birth weight and estimated AML dose via breast milk were 2170 g and 4.2 µg/kg, respectively. The median RID of AML was 4.2% (IQR, 3.1–7.3%). No circulatory disorders were diagnosed in the breastfed infants during the observation period. In summary, the median milk/plasma concentration ratio of AML was 0.85 in breastfeeding women. However, the observed RID of AML in most newborns was less than 10%.

(a) Plasma concentration and (b) milk concentration. The bars represent the median level (—) with 25th (- - - -) and 75th (·····) percentiles of each value.

The bars represent the median level (—) with 25th (- - - -) and 75th (·····) percentiles of each value.
Our achievements described in the present review can be summarized as follows.6,7,10,12,13) 1) Cachectic cancer patients exhibited high plasma concentrations of oxycodone via the decrease in CYP3A activity. The metabolic reduction in cachectic cancer patients was potentially related to the elevated serum level of IL-6. 2) DRD2 genetic mutations and being female led to poor antiemetic efficacy for OINV in PCZ-treated patients. The OPRM1 wild genotype, in addition to being female and having high plasma concentration of PCZ, increased prolactin secretion during oxycodone treatment. 3) RA patients with a genetic mutation of ABCB1 had high blood concentrations of tacrolimus and its 13-O-demethylate. The ABCB1 genetic mutation and its related high blood concentration of tacrolimus decreased kidney function. 4) Chronic inflammation increased the plasma VRCZ concentration via its poor metabolism, whereas it did not alter the plasma ITCZ concentration. Although co-administration of prednisolone did not affect the plasma concentration of triazole antifungal agents, it weakly increased VRCZ metabolism. 5) In breastfeeding women, the median milk/plasma concentration ratio of AML was 0.85. However, the observed RID of AML in most newborns was less than 10%.
I would like to express my thanks to Dr. Junichi Kawakami, Dr. Yasuaki Mino, Dr. Tatsuya Yagi, Dr. Yoshiaki Takashina, Dr. Takahiro Yamada, Dr. Masaki Tashiro, Dr. Hikaru Sato, Ms. Naoko Kubono, Ms. Satoe Harauchi (Department of Hospital Pharmacy, Hamamatsu University School of Medicine), Dr. Kazunori Ohnishi (Oncology Center, Hamamatsu University School of Medicine), Dr. Noriyoshi Ogawa (Department of Rheumatology, Hamamatsu University School of Medicine), Dr. Hiroaki Itoh (Department of Obstetrics and Gynecology, Hamamatsu University School of Medicine), and Dr. Yoshiyuki Kagawa (Department of Clinical Pharmaceutics and Pharmacy Practice, University of Shizuoka). The studies reviewed were supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant numbers JP22926007, JP23790181, JP23926010, JP25928012, JP26460194, and JP26927005.
The author declares no conflict of interest.
This review of the author’s work was written by the author upon receiving the 42nd Sato Memorial Domestic Award.