2020 Volume 43 Issue 4 Pages 688-692
2020 Volume 43 Issue 4 Pages 688-692
Sesamin is a major lignan in sesame seeds, and a recent meta-analysis of controlled trials indicated that sesamin intake decreases blood pressure. The antihypertensive effect of sesamin has been suggested to be due to sesamin-mediated suppression of 20-hydroxyeicosatetraenoic acid production catalyzed by CYP4F2. However, the detailed mechanism underlying inhibition of CYP4F2 function by sesamin remains unclear. In this study, the effects of sesamin on catalytic activity of CYP4F2 were investigated in vitro. Sesamin inhibited luciferin-4F2/3 O-dealkylase activity of recombinant human CYP4F2 with an IC50 value of 0.381 µM. When preincubated in the presence of reduced nicotinamide adenine dinucleotide phosphate (NADPH) for 20 min, sesamin potentiated the inhibition of CYP4F2 activity. Moreover, kinetic analysis of the inactivation revealed that sesamin showed a preincubation time- and concentration-dependent inhibition of CYP4F2 activity yielding a maximal inactivation rate constant (kinact) value of 0.354 min−1 and half-maximal inhibitory concentration (KI) value of 1.12 µM. The inactivation of CYP4F2 by sesamin required NADPH. These results indicated that sesamin is a mechanism-based inactivator of human CYP4F2.
The human CYP4 family is comprised of six subfamilies, CYP4A, CYP4B, CYP4F, CYP4V, CYP4X, and CYP4Z, which typically catalyze ω-hydroxylation of fatty acids and eicosanoids.1) CYP4F2, one of the human CYP4F isoforms, catalyzes ω-oxidation of arachidonic acid and leukotriene B4 in the liver and kidneys.2–4) In addition, this enzyme is capable of ω-hydroxylating vitamin E and vitamin K1.5,6) CYP4F2 is also involved in the oxidative metabolism of a small portion of drugs, such as fingolimod.7)
Although the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE) from arachidonic acid is mainly catalyzed by CYP4F2 and CYP4A11 in the liver and kidney, the contribution of CYP4F2 to this production is suggested to be similar to or greater than that of CYP4A11.2,3) 20-HETE acts as a vasoconstrictor in renal, cerebral, and mesenteric arteries.8) In the renal proximal tubule, on the other hand, 20-HETE reduces blood pressure by inhibiting sodium reabsorption.8) These findings suggest that 20-HETE exhibits different effects on blood pressure depending on its site of production.
Sesame seeds have been commonly consumed as dietary and/or medicinal plant-derived products all over the world. The major lignans in sesame seeds are sesamin and sesamolin. Among these lignans, sesamin accounts for 40–70% of the sesame seed lignans.9) Sesamin has been shown to have several biological effects, including antihypertensive, antitumor, and antioxidative effects.10–12) A recent meta-analysis of controlled trials indicated that sesame consumption reduces systolic blood pressure and/or diastolic blood pressure.13) It has been reported that 5-week administration of 25 g/d of sesame (approximately 50 mg/d of sesame lignan) to overweight subjects decreased 20-HETE by 28% in plasma and 32% in urine in comparison with the control group.14) Furthermore, sesamin inhibited the formation of 20-HETE by human liver microsomes, human renal microsomes, and recombinant human CYP4F2 in vitro.14) These findings suggest that the hypotensive effect of sesamin may be due to inhibition of CYP4F2-mediated 20-HETE synthesis. However, the detailed mechanism underlying sesamin-mediated inhibition of CYP4F2 function is unknown.
In the present study, the effects of sesamin on the catalytic activity of human CYP4F2 were examined in vitro. Here we report that sesamin inactivates human CYP4F2 activity.
Baculovirus-infected insect cell microsomes expressing human CYP4F2 with human reduced nicotinamide adenine dinucleotide phosphate (NADPH)-CYP reductase and cytochrome b5 (Supersomes™) were obtained from Corning Incorporated (Woburn, MA, U.S.A.). Luciferin-4F2/3, luciferin detection reagent, and beetle luciferin were purchased from Promega (Madison, WI, U.S.A.). Other chemicals were obtained from the following sources: sesamin was from Adooq Bioscience (Irvine, CA, U.S.A.); nicotinamide adenine dinucleotide phosphate (NADP), glucose 6-phosphate, and glucose 6-phosphate dehydrogenase were from Oriental Yeast Co., Ltd. (Tokyo, Japan). All other chemicals and solvents used were of the highest quality commercially available.
Inhibition StudiesThe luciferin-4F2/3 O-dealkylase activity of recombinant CYP4F2 was determined according to our previous report,15) with a minor modification. Briefly, an incubation mixture consisted of CYP4F2 Supersomes™ (20 pmol/mL), potassium phosphate buffer (50 mM, pH 7.4), luciferin-4F2/3 (5 µM), sesamin (0–2.5 µM), and an NADPH-generating system (0.5 mM NADP, 10 mM glucose 6-phosphate, 10 mM magnesium chloride, and 1 unit/mL glucose-6-phosphate dehydrogenase) in a final volume of 200 µL. For enzyme kinetics of sesamin-mediated CYP4F2 inhibition, luciferin-4F2/3 at six different concentrations (1–10 µM) was added to incubation mixtures containing four different concentrations of sesamin (0–0.5 µM). Reactions were incubated at 37°C for 20 min. Luminescence was determined using a Sirius luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany). Sesamin was dissolved in dimethylsulfoxide (DMSO) and the final DMSO concentration was adjusted to 0.5% in all samples. The IC50 value was calculated by nonlinear regression analysis with Origin 7.5 J software (OriginLab, Northampton, MA, U.S.A.), using the logistic dose-response. To presume the mode of inhibition, enzyme kinetic data were fitted to the Hill equation by nonlinear regression analysis with Origin 7.5 J software (OriginLab). The apparent Ki value derived from a mixed-type inhibition model of Michaelis–Menten kinetics was calculated by nonlinear regression analysis with GraphPad Prism 7.02 (GraphPad Software Inc., San Diego, CA, U.S.A.).
Inactivation StudiesTo elucidate the ability of sesamin to cause metabolism-dependent CYP4F2 inhibition, we conducted inhibition experiments with or without preincubation as described previously,16) with minor modifications. Briefly, a preincubation mixture consisted of CYP4F2 Supersomes™ (20 pmol/mL), potassium phosphate buffer (50 mM, pH 7.4), sesamin (0–2.5 µM), and the NADPH-generating system in a final volume of 180 µL. After preincubations were performed for 0 min or 20 min, a 20 µL of luciferin-4F2/3 solution was added to the preincubation mixture (final substrate concentration 5 µM). The luciferin-4F2/3 O-dealkylase activity was measured according to the above-mentioned method.
The kinetics of sesamin-mediated CYP4F2 inactivation was determined according to our previous report,16) with minor modifications. Briefly, a preincubation mixture consisted of CYP4F2 Supersomes™ (200 pmol/mL), potassium phosphate buffer (50 mM, pH 7.4), sesamin (0–1 µM), and the NADPH-generating system in a final volume of 200 µL. Preincubations were conducted at 37°C for 0, 2, 4, and 6 min. A 20 µL of aliquot was removed from the preincubation mixtures at the indicated time points and transferred to 180 µL of enzyme assay mixture including luciferin-4F2/3 and the NADPH-generating system. Incubations were carried out and the luciferin-4F2/3 O-dealkylase activity was determined according to the same method as described above. The maximal inactivation rate constant (kinact) and half-maximal inhibitory concentration (KI) were calculated by nonlinear regression analysis using GraphPad Prism 7.02 (GraphPad Software Inc.).
To evaluate the effect of NADPH on the inactivation of CYP4F2 by sesamin, the preincubation mixture was prepared according to the same method as kinetic analysis of the inactivation. Preincubations were performed with or without the NADPH-generating system in both the presence and absence of sesamin for 6 min.
Statistical AnalysisData are presented as the mean ± standard deviation (S.D.) of triplicate determinations. The statistical significance of differences between the means of the various groups was assessed by one-way ANOVA followed by Dunnett’s or Bonferroni’s post hoc test. All statistical analyses were conducted with the GraphPad Prism 7.02 (GraphPad Software Inc.).
The ability of sesamin to inhibit CYP4F2 activity was investigated with recombinant human CYP4F2. CYP4F2 activity was concentration-dependently inhibited by sesamin with an IC50 value (mean ± S.D.) of 0.381 ± 0.031 µM (Fig. 1). To identify the mode of CYP4F2 inhibition by sesamin, we conducted kinetic analysis of the inhibition. First, luciferin-4F2/3 O-dealkylation by recombinant human CYP4F2 was fitted to the Hill model (Fig. 2). In this reaction, Vmax values decreased and S50 values increased with increasing sesamin concentration, whereas the Hill coefficients showed no substantial changes (Table 1). These results indicated that sesamin showed mixed-type inhibition. As an inhibition model of Hill kinetics was not available, the apparent Ki value was calculated from the mixed-type inhibition model of Michaelis–Menten kinetics and had a mean ± S.D. of 0.272 ± 0.041 µM.

Results are expressed as the percentage of the control activity. Each point and bar represent the mean ± S.D. of 3 independent experiments.

Results are expressed as the catalytic activities at the indicated substrate concentrations. Each point and bar represent the mean ± S.D. of 3 independent experiments.
| Sesamin concentrations (µM) | Vmax (pmol/min/nmol P450) | S50 (µM) | n |
|---|---|---|---|
| 0 | 49.0 ± 5.9 | 3.38 ± 0.40 | 2.04 ± 0.01 |
| 0.125 | 41.4 ± 2.0 | 3.93 ± 0.24 | 1.89 ± 0.06* |
| 0.25 | 33.8 ± 1.6** | 4.17 ± 0.33 | 1.94 ± 0.06 |
| 0.5 | 24.4 ± 2.2*** | 4.45 ± 0.53* | 1.93 ± 0.07 |
Values of Vmax, S50 (substrate concentration to give 50% of Vmax), and n (Hill coefficient for cooperative binding) are the means ± S.D. of 3 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control (Dunnett’s test).
The effects of preincubation on sesamin-mediated CYP4F2 inhibition were investigated to evaluate whether sesamin shows a metabolism-dependent inhibition of CYP4F2 activity. The CYP4F2 inhibition was enhanced by 20-min preincubation of sesamin in the presence of NADPH (Fig. 3). The IC50 values (mean ± S.D.) with and without preincubation were 0.0634 ± 0.0106 and 0.399 ± 0.013 µM, respectively.
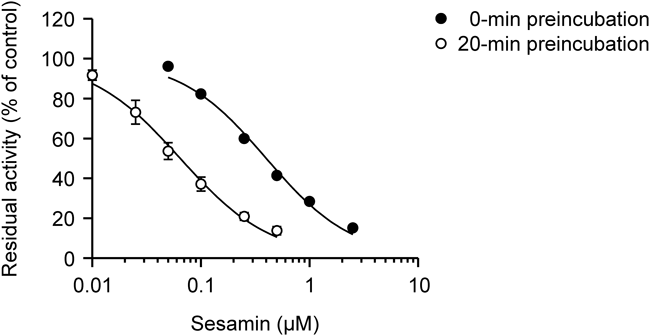
Results are expressed as the percentage of the corresponding control activities. Each point and bar represent the mean ± S.D. of 3 independent experiments.
Kinetic analysis of inactivation was performed to characterize the parameters for inactivation of CYP4F2 by sesamin. CYP4F2 activity was decreased by sesamin in a preincubation time- and concentration-dependent manner with kinact and KI values (mean ± S.D.) of 0.354 ± 0.020 min−1 and 1.12 ± 0.05 µM, respectively (Fig. 4).

(A) Results are expressed as the percentage of the control activity at the indicated preincubation time points. (B) Results are expressed as the observed rate of CYP4F2 inactivation (kobs) calculated from the initial slopes of the linear regression lines of semilogarithmic plots (A). Each point and bar represent the mean ± S.D. of 3 independent experiments.
To determine requirement for sesamin metabolism in the CYP4F2 inactivation, the preincubation of sesamin was conducted in the presence and absence of NADPH. Preincubation of recombinant CYP4F2 with sesamin or NADPH alone did not change CYP4F2 activity (Fig. 5). On the other hand, the presence of both sesamin and NADPH significantly decreased this activity.

Results are expressed as the percentage of the control activity at the preincubation time of 6 min. Each column and bar represent the mean ± S.D. of 3 independent experiments. The statistical significance was evaluated by Bonferroni’s test.
In this study, we focused on the effects of sesamin on CYP4F2 activity. The results of the present study revealed that sesamin efficiently inhibited luciferin-4F2/3 O-dealkylase activity of recombinant CYP4F2. It has been reported that sesamin at a concentration of 1 µM shows strong inhibition of tocopherol ω-hydroxylation by recombinant CYP4F2.5) In addition, sesamin has been shown to inhibit arachidonic acid ω-hydroxylase activity of recombinant CYP4F2 in a concentration-dependent manner (IC50 = 1.87 µM).14) These findings indicated that sesamin has the ability to inhibit CYP4F2 activity regardless of the substrate used. Furthermore, in this study, we demonstrated that sesamin inactivated CYP4F2. This inactivation showed a preincubation time- and concentration-dependent manner and required NADPH during preincubation. These results suggest that the sesamin-mediated inactivation of CYP4F2 may proceed via metabolism of this compound. At present, the chemical structure of a metabolic intermediate of sesamin leading to the CYP4F2 inactivation remains unclear. It has been reported previously that sesamin is a mechanism-based inhibitor of CYP2C9.17) The underlying mechanism has been considered to involve quasi-irreversible inhibition by formation of a metabolic intermediate complex of the CYP2C9 heme iron and a carbene generated by CYP2C9-mediated oxidation of the methylenedioxyphenyl group of sesamin.18) This complex has been suggested to be dissociated in a relatively short time with a half-life of about 10 min and CYP2C9 then returns to the active form.18) Sesamin-mediated inactivation of CYP4F2 may occur by a similar mechanism. Further studies are needed to elucidate the detailed mechanism.
The CYP4F2 inhibitors reported to date are 17-octadecynoic acid (17-ODYA)3,15,19) and HET0016.15,20) 17-ODYA was first characterized as a suicide inhibitor of leukotriene B4 ω-hydroxylase including CYP4F in polymorphonuclear leukocytes.21) On the other hand, HET0016 was developed as a selective inhibitor of arachidonic acid ω-hydroxylase.22) HET0016 has been reported to be an irreversible inhibitor of rat CYP4A1.23) However, the mechanism underlying the inhibition of CYP4F2 by these chemical inhibitors has not yet been clarified. To our knowledge, sesamin is the first compound to inactivate CYP4F2. The inactivation potency (kinact/KI) of sesamin against CYP4F2 (316 L/mmol/min) is 2.3-fold higher than that of CYP2C9 (138 L/mmol/min).17) This result indicates that sesamin is a potent mechanism-based inactivator of CYP4F2.
It has been reported that sesamin inhibits CYP1A2 and CYP3A4 activity,17) in addition to CYP4F2 and CYP2C9. Inhibition of CYP1A2 and CYP3A4 by sesamin is reversible and competitive.17) The inhibitory potencies (Ki) of sesamin against CYP1A2 and CYP3A4 are 75 and 4.2 µM, respectively, indicating CYP isoform selectivity.17) Therefore, we found that sesamin inhibits CYP4F2 activity much more potently than CYP1A2 and CYP3A4 activity, although the apparent Ki for CYP4F2 (0.272 µM) was calculated in this study.
A previous controlled trial with mildly hypertensive subjects showed that administration of 60 mg/d of sesamin for 4 weeks significantly reduced blood pressure by an average of 3.5 mmHg systolic blood pressure and 1.9 mmHg diastolic blood pressure in comparison with the placebo control group.10) These findings and our results suggest that sesamin intake may reduce blood pressure by potent inhibition of hepatic and/or renal CYP4F2-mediated 20-HETE production. However, it is difficult to take such large quantities of sesame in daily life. In Japan, the daily intake of sesamin is recommended to be 10 mg in commercially available nutritional supplements. Therefore, the contribution of CYP4F2 inhibition to the antihypertensive effect of sesamin may be limited.
In conclusion, we demonstrated that sesamin is a mechanism-based inactivator of human CYP4F2.
This work was supported in part by a Grant-in-Aid for Encouragement of Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17H00484).
The authors declare no conflict of interest.